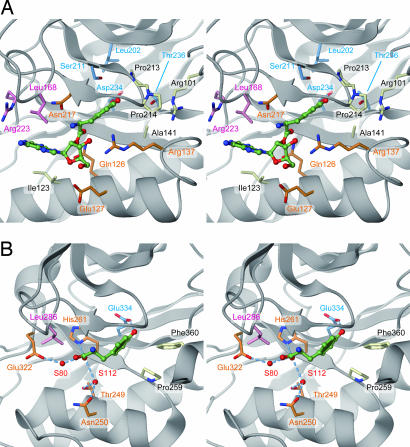
Fig. 5.
Model of Tyr-tRNAPhe recognition by archaeal/eukaryal PheRS-β. (A) The editing pocket of P. horikoshii PheRS-β. The posttransfer substrate (Tyr-A76, green) was placed on the basis of the structure of T. thermophilus PheRS complexed with tyrosine. The residues shown are proposed to participate in Tyr-A76 recognition. Light blue, residues close to the Tyr hydroxy group; pink, residues close to the A76 moiety; orange, residues close to the Tyr-A76 ester bond; white, residues not mutated. (B) The T. thermophilus PheRS editing pocket, bound with tyrosine for comparison. Water molecules are depicted by red spheres. Dashed lines indicate hydrogen-bonding interactions.