Abstract
Listeria monocytogenes is a facultative intracellular gram-positive bacterium responsible for severe opportunistic infections in humans and animals. We had previously identified a gene encoding a putative UDP-N-acetylglucosamine 2-epimerase, a precursor of the teichoic acid linkage unit, in the genome of L monocytogenes strain EGD-e. This gene, now designated lmo2537, encodes a protein that shares 62% identity with the cognate epimerase MnaA of Bacillus subtilis and 55% identity with Cap5P of Staphylococcus aureus. Here, we addressed the role of lmo2537 in L. monocytogenes pathogenesis by constructing a conditional knockout mutant. The data presented here demonstrate that lmo2537 is an essential gene of L. monocytogenes that is involved in teichoic acid biogenesis. In vivo, the conditional mutant is very rapidly eliminated from the target organs of infected mice and thus is totally avirulent.
Listeria monocytogenes is a gram-positive bacterium that is widespread in nature and responsible for sporadic severe infections in humans and other animal species (63). This pathogen is a facultative intracellular microorganism capable of invading most host cells, including epithelial cells, hepatocytes, fibroblasts, endothelial cells, and even macrophages. Each step of intracellular parasitism by L. monocytogenes is dependent upon the production of virulence factors (12, 17), and several genes are specifically induced during host cell infection. In particular, most PrfA-regulated virulence genes are more efficiently expressed during intracellular growth (10, 11, 22, 34, 45).
Several “genome-scale” genetic methods have been developed recently to discover new genes involved in bacterial pathogenesis (for reviews, see references 4, 44, and 53). Among them, in vivo expression technology (IVET) (41) allows the identification of genes whose expression is regulated (generally up) during infection. This approach requires subsequent gene inactivation to evaluate the precise role of the in vivo-regulated genes in bacterial virulence. IVET has been adapted to L. monocytogenes, using either the hly gene, encoding listeriolysin O (LLO) or the green fluorescence protein (GFP) gene, as reporter genes (16, 25, 67). We had previously identified by this approach, a locus now designated lmo2537 (26), as one of the in vivo-inducible genes of L. monocytogenes (16). This gene encodes a putative UDP-N-acetylglucosamine 2-epimerase (UDP-GlcNAc 2-epimerase) involved in teichoic acid (TA) biosynthesis (31, 32, 47).
The UDP-GlcNAc 2-epimerase activities of B. subtilis MnaA (61) and Staphylococcus aureus Cap5P (33) proteins have been experimentally demonstrated. The two proteins, which share 58% amino acid identity, allow the formation of UDP-N-acetylmannosamine from UDP-GlcNAc, a precursor required for TA linkage unit synthesis (32). The linkage unit is the acceptor for the polymerization of the main TA chain (42). After their translocation across the cytoplasmic membrane through an ABC transporter (38), polymerized TA is covalently attached to the peptidoglycan via this linkage unit. The TA of L. monocytogenes is a poly(ribitol phosphate) carrying glycosidic substitutions on ribitol residues (19, 24, 30).
Listeria strains can be classified by their antigenic properties on the basis of their somatic (O) and flagellar (H) antigens (57), and TAs constitute the major somatic antigens. Whereas in serogroup 1/2 the poly(ribitol phosphate) of TA is substituted with GlcNAc and rhamnose; in serogroup 4 the GlcNAc is incorporated in the TA chains. TA-associated glycosidic substituents have been shown to serve as receptor for phages in L. monocytogenes serotype 1/2a (66).
In B. subtilis, the construction and characterization of knockout and conditional mutants in several genes of the TA biosynthetic pathway have established that TA is essential for cell viability (see references 43 and 61 and references therein). Notably, the gene mnaA (encoding the UDP-GlcNAc 2-epimerase activity) has been experimentally shown to be essential (61), whereas its S. aureus counterpart, cap5P, is not essential for bacterial survival because of gene redundancy (33). Moreover, a recent report demonstrated that TA is not essential in S. aureus (64). Indeed, when the putative N-acetylglucosamine-1-phosphate transferase gene tagO involved in the initial step of linkage unit synthesis was deleted from S. aureus, the mutant strain remained viable under laboratory conditions despite TA deficiency. Remarkably, the mutation affected the nasal colonization of S. aureus and impaired adherence to nasal cells (64), revealing a direct role of TA in S. aureus pathogenicity. The authors of that study showed very recently that TA was also involved in the induction and the progression of endovascular S. aureus infection, possibly through a specific interaction with endothelial cells (65).
Thus, different species have different requirements for TA, suggesting that this polymer may display different functions. These observations prompted us to evaluate the function of lmo2537 in L. monocytogenes viability and pathogenesis. We constructed and characterized a conditional chromosomal deletion mutant and provided evidence that lmo2537 is an essential gene. To date, this is the first essential gene experimentally identified in L. monocytogenes.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Brain heart infusion (BHI; Difco Laboratories) and Luria-Bertani broth and agar (Difco Laboratories, Detroit, Mich.) were used to grow L. monocytogenes and Escherichia coli strains, respectively. Wild-type EGD-e was transformed by electroporation as previously described (5). Strains harboring plasmids were grown in the presence of the following antibiotics: erythromycin at 200 μg ml−1 (E. coli) and at 5 μg ml−1 (L. monocytogenes), ticarcillin at 100 μg ml−1, kanamycin at 50 μg ml−1, and chloramphenicol at 10 μg ml−1. A list of strains and plasmids used in this study is presented in Table 1.
TABLE 1.
L. monocytogenes strains and plasmids used in this study
| Listeria plasmid or strain | Genotype, description, or sequence | Resistance marker(s)a | Source or reference |
|---|---|---|---|
| Plasmids | |||
| PCR | Gram-negative cloning vector | Ap, Km | Invitrogen |
| pCR -lmo2537 | pCR with lmo2537 | Ap, Km | This study |
| pLIV1 | Gram-negative/gram-positive shuttle vector; thermosensitive | Cm, Em | 15 |
| pLIV-lmo2537 | pLIV1 with lmo2537 | Cm, Em | This study |
| pG1ts | Gram-negative/gram-positive shuttle vector; thermosensitive | Em, Km | P. Trieu-Cuot |
| pG1ts -Δlmo2537 | pG1ts with lmo2537 flanked regions | Em, Km | This study |
| pATgfp7 | pAT28 carrying the gfp gene under psvpA control | Spc | 48 |
| pATgfp/plmo2537 | pAT28 carrying the gfp gene under plmo2537 control | Spc | This study |
| Strains | |||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen | |
| L. monocytogenes | |||
| EGD-e | Virulent wild-type clinical isolate, serovar 1/2a | 26 | |
| EGDpGΔlmo2537-lmo2537 | EGD-e carrying a chromosomally integrated copy of pG1tsA-Km-B | Em, Cm, Km | This study |
| EGD lmo2537/pLiv-lmo2537 | EGD-e carrying a chromosomal deletion of lmo2537 and plasmid pLIV-lmo2537 | Em, Cm, Km | This study |
Cm, chloramphenicol; Em, erythromycin; Ap, ampicillin; Spc, spectinomycin; Km, kanamycin.
Genetic manipulations.
Total DNA from L. monocytogenes cells was prepared as described previously (5). Standard techniques were used for plasmid DNA preparation, fragment isolation, DNA cloning, and restriction analysis (56). Restriction enzymes and ligase were purchased from New England Biolabs and used as recommended by the manufacturer. DNA was amplified with Taq DNA polymerase (Promega) for 35 cycles of 60 s at 95°C, 60 s at 55°C, and 60 s at 72°C in a GeneAmp system 9600 thermal cycler (Perkin-Elmer). Oligonucleotides were synthesized by Eurogentec. Nucleotide sequencing was carried out with Taq dideoxy terminators and by the DyePrimer cycling sequence protocol developed by Applied Biosystems (Perkin-Elmer) with fluorescently labeled dideoxynucleotides and primers, respectively. Fluorescently labeled primers were purchased from Life Technologies. Labeled extension products were analyzed on an ABI Prism 310 apparatus (Applied Biosystems/Perkin-Elmer). Protein and nucleotide databases were searched by using the programs BLASTP and BLASTN (National Center for Biotechnology Information, Los Alamos, NM), which are available via the internet. Protein sequences were aligned by using the program CLUSTAL W (http://www.infobiogen.fr/services/analyseq/cgi-bin/clustalw_in.pl).
Construction of a conditional Δlmo2537 chromosomal mutant.
First, we tried to construct a chromosomal deletion mutant by allelic replacement, using the procedure classically used in our laboratory (48). Briefly, the two DNA regions flanking lmo2537 were amplified by PCR using L. monocytogenes EGD-e genomic DNA (see the list of primers in Table 2). The 416-bp upstream fragment (designated A), immediately preceding the ATG of lmo2537, was flanked with EcoRI and KpnI restriction sites. The 499-bp downstream fragment (designated B), immediately following the stop codon of lmo2537, was flanked with BamHI and PstI restriction sites. After digestion, the two fragments A and B were successively cloned into the corresponding sites of the thermosensitive pG1ts shuttle vector. This plasmid (kindly provide by P. Trieu-Cuot, Institut Pasteur, Paris, France) is a derivative of plasmid pG+host5 (8), carrying the promoterless and terminatorless kanamycin resistance cassette aphA-3 (Km) cloned into the KpnI and BamHI sites of pG+host5. Insertion of fragment A between the EcoRI and KpnI sites (upstream of the aphA-3 gene) and of fragment B between the BamHI and PstI sites (downstream of the aphA-3 gene) of pG1ts yielded plasmid pG1tsA-Km-B. This recombinant plasmid was then introduced into L. monocytogenes EGD-e by electroporation, and the transformants were selected for erythromycin resistance (Emr) at 30°C. Integration of pG1tsA-Km-B in the chromosome of EGD-e was realized (via a single crossover in either the A or the B fragment) after passage at a nonpermissive temperature (37°C) in the presence of kanamycin, yielding the merodiploid strain designated EGDpGΔlmo2537-lmo2537. We then screened the second crossover event, leading to the excision of the plasmid carrying the wild-type lmo2537 allele, by growing the recombinant bacteria at permissive temperature (30°C) for many generations in the absence of Emr selection. When selected for kanamycin resistance (Kmr), all of the clones isolated were also Emr, i.e., none of them had lost the integrated plasmid. In contrast, in the absence of Kmr selection, Ems clones were obtained, but all of them were also Kms, indicating that the second crossover (plasmid excision) was possible but only when the wild-type copy of the gene was conserved in the chromosome.
TABLE 2.
Primers used in this study
| Analysis | Primer
|
|
|---|---|---|
| Sequence | Characteristics | |
| Chromosomal deletion | CGGAATTCAACTGGCGGCGGATCGGCAATTAT | Region A upstream of lmo2537, forward primer |
| GGGGTACCCGCCAACTAGCGGTGCC | Region A upstream of lmo2537, reverse primer | |
| CGGGATCCC GAAGCTGGGACGTTGAAA | Region B downstream of lmo2537, forward primer | |
| AAAACTGCAGACTCACTTATCATGAGCTGCAT | Region B downstream of lmo2537, reverse primer | |
| lmo2537 gene cloning | CCTCTAGAAGGAGGTTCAAAATTGGCTAAAATC | lmo2537, 5′ end |
| CCTCTAGATTACACGATAAAATCTTCTGGGCG | lmo2537, 3′ end | |
| lmo2537 promotor cloning | CGG AAT TCG TTT AGT AGC AGC TCC TGA AGG | Promoter region (260 bp), 5′ end |
| GGG GTA CCT TTT GAA CCT CCT TAT AAA AAA AC | Promoter region (260 bp), 3′ end | |
| RT-PCR | GGACTTAGAACATGGAATAAATATTCGCCG | Within lmo2537, forward primer |
| CTGCGGTGAGCCGTCATAAGTATTAAACGATTG | Within lmo2537, reverse primer | |
| Real-time PCR | GGCGCAAGCAGCTAATCC | Within lmo2537, forward primer |
| GTGACTCTTGATTGCTGCTAAAA | Within lmo2537, reverse primer | |
| AAATGCGGACATCATTCCTAGACT | Within gyrA, forward primer | |
| TTTAACCCGTCACGAACATCAG | Within gyrA, reverse primer | |
Expression of lmo2537 under SPAC promoter control.
The wild-type lmo2537 gene preceded by its ribosome-binding site was amplified by PCR from L. monocytogenes (EGD-e) genomic DNA using the primer pair 5′-CCT CTA GAA GGA GGT TCA AAA TTG GCT AAA ATC-3′ (the Shine-Dalgarno sequence is underlined) and 5′-CCT CTA GAT TAC ACG ATA AAA TCT TCT GGG CG-3′. The primers were designed to generate cohesive XbaI restriction sites (indicated in italics). The amplified double-stranded DNA fragments were first cloned into the pCR cloning vector by using the TA cloning kit (Invitrogen Corp., San Diego, CA). The XbaI-XbaI fragment carrying the entire gene lmo2537 was then subcloned into the XbaI site of the pLIV1 shuttle plasmid (kindly provided by Darren Higgins), located immediately downstream of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible SPAC promoter (15) to yield plasmid pLIV-lmo2537.
Plasmid pLIV-lmo2537 was introduced into strain EGDpGΔlmo2537-lmo2537 (carrying a chromosomally integrated copy of pG1tsA-Km-B) by electroporation and selection for the acquisition of pLIV1-encoded chloramphenicol resistance (Cmr) at 30°C and in the presence of 1 mM IPTG (the recombinant clones were thus Emr, Kmr, and Cmr). Excision of the chromosomally integrated pG- recombinant plasmid, leading the substitution of the wild-type lmo2537 allele by the Kmr cassette, was then screened after repeated growth at 30°C in the presence of kanamycin (to keep the mutated chromosomal allele), chloramphenicol, and 1 mM IPTG (to keep the pLIV recombinant plasmid and allow cell viability) but in the absence of erythromycin (to allow loss of pG1). This procedure allowed the spontaneous excision of the pG- recombinant plasmid carrying the wild-type allele (via a unique crossover). The resulting conditional mutant (designated EGDΔlmo2537/pLiv-lmo2537) carries a chromosomal deletion of lmo2537 and a plasmid-borne wild-type lmo2537 allele under IPTG-inducible promoter control.
All of the constructions were confirmed by PCR sequence analysis of chromosomal DNA from the mutants.
Cloning of GFP under plmo2537promoter control.
We used the Pro5′Eco (5′-CGG AAT TCG TTT AGT AGC AGC TCC TGA AGG-3′) and Pro3′Kpn primers (5′-GGG GTA CCT TTT GAA CCT CCT TAT AAA AAA AC-3′), which are flanked by EcoRI and KpnI restriction sites, to amplify the 260-bp promoter region of lmo2537. The resulting amplified fragment was cloned as an EcoRI/KpnI insert in the listerial vector pATgfp7 (48) by restriction fragment exchange, using EcoRI and KpnI, creating pATgfplmo2537.
Kinetics of bacterial growth in broth.
The conditional mutant strain EGDΔlmo2537/pLiv-lmo2537 was first grown overnight at 30°C in BHI medium supplemented with kanamycin, chloramphenicol, and 1 mM (final) IPTG. Bacteria were then collected by centrifugation and resuspended in fresh medium without IPTG. The bacterial suspension was finally diluted 10-fold into BHI medium containing kanamycin, chloramphenicol, and various concentrations of IPTG (1 mM, 100 μM, or 10 μM or no IPTG).
Electron microscopy.
After an overnight growth at 30°C in BHI medium supplemented with 1 mM (final) IPTG (and the appropriate antibiotics), strains EGDΔlmo2537/pLiv-lmo2537 and EGD-e were collected by centrifugation and resuspended in fresh medium without IPTG. The bacterial suspensions were then diluted 10-fold into BHI medium containing either 1 mM IPTG or no IPTG (and the appropriate antibiotics) and reincubated at 30°C with agitation. After 6 h of incubation, bacteria were collected and processed for observation under the electron microscope by using a thin-sectioning procedure (46).
Protein preparation and analyses.
Bacteria were grown in BHI containing either 1 mM or 50 μM IPTG (for the conditional mutant) or no IPTG (for EGD-e). After several hours of growth with agitation at 30°C, the bacteria were collected by centrifugation and adjusted to the same final optical density at 600 nm (OD600) of 0.8. Envelope proteins were solubilized according to a previously described procedure (28). Briefly, bacterial pellets were washed twice in phosphate-buffered saline and resuspended in an isosmotic sucrose solution (sucrose, 250 mM; Tris-HCl [pH 7.5], 10 mM; EDTA, 0.4 mM). After centrifugation at 7,000 × g, the pellet was finally resuspended in 1% sodium dodecyl sulfate (SDS)-100 mM Tris-HCl and heated at 95°C before sonication. Samples were cooled on ice for 5 min before the addition of solubilization buffer (urea, 8 M; thiourea, 2.5 M; CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 4%; dithiothreitol, 50 mM; spermine, 6.25 mM; vanadate, 1 mM). After 1 h of incubation on a rotating wheel, the samples were centrifuged for 40 min at 40,000 rpm (in a Beckman-Coulter ultracentrifuge). Fractions of the supernatant were then stored at −80°C. Each well corresponds to 3 ml of bacterial culture (adjusted to a final OD600 of 0.7).
Cell-free supernatants were filtered through a 0.22-μm-pore-size Millipore filter. The filtered supernatants were concentrated by trichloroacetic acid (TCA) precipitation. After being washed with acetone, the pellets were resuspended into loading buffer (adjusted to the same final OD600 of bacteria). Electrophoresis was carried out as described previously (9) in 11% SDS-polyacrylamide gels (Bio-Rad). Proteins present in envelope fractions were stained with Coomassie blue. Proteins present in culture supernatants were revealed by silver staining.
Estimation of cell wall phosphate.
Cell walls were prepared essentially as previously described (60). Briefly, strains EGDΔlmo2537/pLiv-lmo2537, EGD-e, and EGD-pLIV were first grown overnight in BHI medium (supplemented with appropriate antibiotics) and 1 mM IPTG. Bacterial cultures were then centrifuged, washed with the IPTG-free medium, and resuspended in parallel in fresh IPTG-free and IPTG-containing medium to an OD600 of 0.075. Cultures of strain EGD-e contained no IPTG. Cell walls were isolated from the cultures at an OD600 of 1.3 as previously described (60), except that SDS was used at a concentration of 4% instead of 3%. Lyophilized cell walls were mineralized (2), and the phosphate concentration was determined according to the method of Chen (13).
Antibiotic sensitivity assays.
The conditional mutant was initially grown in BHI-chloramphenicol (BHI-Cm) containing 1 mM IPTG and plated onto BHI-Cm containing a limiting amount of IPTG (10 μM [which is sufficient to promote growth]). Agar disk diffusion test was used to monitor comparative antibiotic susceptibilities on Mueller-Hinton solid medium. The mutant showed an increased susceptibility to bacitracin, colistin, and polymyxin. The following antibiotics were tested: clindamycin, erythromycin, pristinamycin, ofloxacin, chloramphenicol, rifampin, fosfomycin, minomycin, gentamicin, kanamycin, vancomycin, teicoplanin, bacitracin, colistin, and polymyxin. The sensitivity pattern of the mutant (supplemented with 10 μM IPTG) to the first 12 of these antibiotics (i.e., clindamycin to teicoplanin) was similar to that of EGD-e (except the resistances inherited upon its construction).
Analysis of cell wall integrity.
To examine the susceptibility of the conditional mutant to lysis after incubation with a cell wall hydrolase, mutanolysin was used to assess the integrity of the cell wall. Briefly, bacteria were first grown overnight in BHI medium (or BHI containing chloramphenicol and kanamycin and supplemented with 1 mM IPTG for the conditional mutant). Bacteria were then collected by centrifugation and resuspended into fresh medium without IPTG. For the conditional mutant, the bacterial suspension was finally diluted 20-fold into BHI medium containing kanamycin and chloramphenicol and either supplemented or not supplemented with 1 mM IPTG (EGD-e was diluted in BHI medium). Growth was pursued for ca. 6 h at 30°C with agitation. Bacterial cultures were then adjusted to a final OD600 of 1 with BHI, collected by centrifugation, and resuspended in lysis buffer (50 mM NaH2PO4 buffer at pH 6.8) containing or not containing mutanolysin (Sigma) at a final concentration of 50 U/ml. Suspensions were incubated at 30°C, and lysis monitored by determining the decrease in the OD550 of the sample over time (at 15-min intervals for 90 min), as previously described (50).
RNA extraction and reverse transcriptase PCR (RT-PCR) assays.
L. monocytogenes was grown in BHI, and bacteria in exponential phase (OD600 from 0.4 to 0.6) were collected by centrifugation. RNA extraction was done as described previously (54). DNA contamination was removed by digestion with DNase I (Roche Diagnostic), and the RNA preparation was purified by using the RNeasy kit (QIAGEN).
For RT-PCR, amplification of lmo2537 cDNA was carried out by using the Superscript II kit (Invitrogen) with the forward primer 5′-GGA CTT AGA ACA TGG AAT AAA TAT TCG CCG-3′ and the reverse primer 5′-CTG CGG TGA GCC GTC ATA AGT ATT AAA CGA TTG-3′.
Infection of macrophages and real-time PCR assays.
Strain EGDΔlmo2537/pLiv-lmo2537 was first grown overnight in BHI medium supplemented with chloramphenicol, kanamycin, and 1 mM IPTG. Bacterial growth in J774 macrophage-like cells was performed as described previously (29) in the absence or presence of gentamicin (50 μg/ml [final]) at a bacterium/macrophage ratio of 10:1. At selected intervals after infection, cells were washed three times and processed to count the infecting bacteria. Cells were lysed by adding cold distilled water, and the number of viable bacteria released from the cells was determined by spreading them onto BHI plates containing 1 mM IPTG. Each experiment was carried out in triplicate and repeated three times.
To extract RNA of L. monocytogenes grown in J774 macrophages, cells were infected at a multiplicity of infection of 20 for 30 min. The cells were then washed three times and reincubated for 30 min or 4 h with gentamicin (10 μg/ml). Cells were lysed with 0.1% Triton X-100, and the supernatant containing the bacteria was centrifuged and washed twice with saline buffer. Bacteria were broken in a solution of TRIzol (1 ml) (Invitrogen) with miniglass beads using a BeadBeater apparatus (Polylabo). RNA was extracted with chloroform-isoamyl alcohol and precipitated with isopropyl alcohol. Total RNA was finally resuspended in diethyl pyrocarbonate-treated water. Contaminating DNA was removed by digestion with DNase I according to the manufacturer's instructions (Roche Diagnostics).
As a control, we monitored lmo2537 expression in Dulbecco modified Eagle medium (DMEM), the medium used for the cellular infection. Bacteria were diluted in DMEM (the same inocula as in the cells) and incubated at 37°C under CO2 for 4.5 h.
After an RT step, real-time PCRs were carried out by using the ABI Prism 7700 sequence detection system. The conditions were identical for all reactions. The 25-μl mixture consisted of 4 μl of template, 12.5 μl of SYBR green jump start Taq ready mix (Sigma), and 5 pmol of each primer. The following pairs of primers were designed (using the primer express software) in order to amplify mRNAs: lmo2537 forward primer, 5′-GGC GCA AGC AGC TAA TCC-3′; lmo2537 reverse primer, 5′-GTG ACT CTT GAT TGC TGC TAA AA-3′; gyrA forward primer, 5-AAA TGC GGA CAT CAT TCC TAG ACT-3′; and gyrA reverse primer, 5′-TTT AAC CCG TCA CGA ACA TCA G-3′. The reactions were carried out in sealed tubes. The results were normalized to the amount of gyrA mRNA.
The gene gyrA was used because we had previously determined that its expression remained constant in BHI (in exponential or stationary phase [6]) and in infected cells (54Catherine Raynaud, unpublished data). Each assay was performed at least in triplicate. Two independent experiments were performed. For each experiment, the infection was performed twice at each time point. For each RNA extract (two per experiment), cDNA quantifications were made in triplicate. Thus, the calculated epi/gyr ratios correspond to the mean of six values (the error bars indicated in the figure correspond to the standard deviation of these six values).
Virulence studies. (i) LD50.
The virulence of the mutant was first estimated by determining the 50% lethal dose (LD50) using the Probit method (55). Animal experiments were performed according to the INSERM guidelines for laboratory animals' husbandry. Specific-pathogen-free, 6- to 8-week-old female Swiss mice (Janvier, Le Genest St. Isle, France) were used. The two strains were grown to stationary phase (in BHI-Cm supplemented with 1 mM IPTG for the conditional mutant) and stored at −80°C in 1-ml fractions. Bacteria were diluted in 0.15 M NaCl and then inoculated intravenously into the mice via the lateral tail vein (0.5 ml). Groups of five mice were challenged with various doses of bacteria (107, 106, 105, and 104 bacteria per mouse), and mortality was observed for 10 days. The LD50 of EGD-e is ca. 104.5 bacteria, while the LD50 of EGDΔlmo2537/pLiv-lmo2537 is ∼4 log10 greater (>108 bacteria [data not shown]).
(ii) In vivo persistence of the conditional mutant.
Groups of five mice were infected intravenously with sublethal doses of either EGDΔlmo2537/pLiv-lmo2537 (2 × 107 bacteria per mouse) or EGD-e (104 bacteria per mouse). Bacterial counts were determined in the spleens and livers at 3 days after infection. Organs (spleens and livers) were aseptically removed and separately homogenized in 0.15 M NaCl. Bacterial counts in organs homogenates were determined at various intervals on BHI agar plates (supplemented with chloramphenicol and 1 mM IPTG for the conditional mutant) as described previously (5).
(iii) Protection.
Mice were immunized intravenously with either 104 CFU of EGD-e or 2 × 107 CFU of EGDΔlmo2537/pLiv-lmo2537. A group of nonimmunized mice was also used as a control. After 28 days, mice from the two groups of primed mice and naive mice were challenged with 105 CFU of EGD-e. The numbers of bacteria in spleens and livers were determined 3 days after challenge on BHI agar plates. The detection limit was 10 CFU per spleen (and 100 CFU per liver).
RESULTS
Organization of the lmo2537 locus.
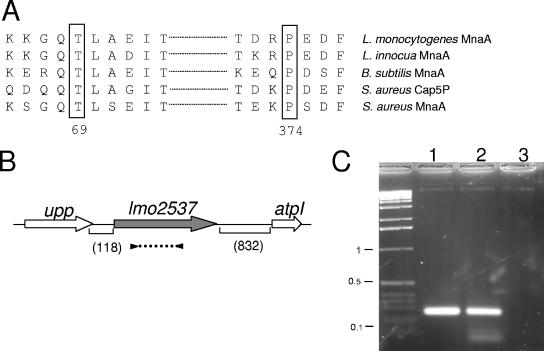
The gene lmo2537 encodes a protein of 379 residues that shares 62% amino acid identity with MnaA of B. subtilis (formerly named YvyH, (59, 61) and 55% identity with Cap5P of S. aureus (33). The gene capP of S. aureus belongs to a cluster of 16 genes that are involved in serotype 5 capsular polysaccharide synthesis. The protein Cap5P has been shown to have UDP-GlcNAc 2-epimerase activity in vitro (33). Inactivation of capP did not provoke any functional defect, most likely due to the presence of a paralogous functional copy of the gene in the S. aureus genome. This paralog, designated mnaA, encodes a protein that has 60% identity with Cap5P and also 60% identity with Lmo2537. Notably, the genetic organization of the mnaA locus of S. aureus is identical to that of lmo2537 (i.e., flanked by the upp and atpI genes). In contrast, B. subtilis carries a unique UDP-GlcNAc 2-epimerase gene, mnaA (61). Mutations in MnaA, resulting in a thermosensitive TA-deficient phenotype, have been identified. The mutant strain carries two amino acid substitutions (T69I and P374L) in the MnaA protein and develops at a nonpermissive temperature (47°C) a coccoid-like morphology similar to that observed for mutants in the tag gene cluster (51, 58). Notably, these two critical residues T69 and P374 are perfectly conserved in the protein Lmo2537 (Fig. 1A). No paralogue of lmo2537 was identified in the genome of L. monocytogenes. Likewise, L. innocua carries also a unique epimerase gene, lin2681, encoding a protein sharing 95% identity with Lmo2537.
FIG. 1.
Schematic organization of the lmo2537 locus. (A) Alignment of the B. subtilis MnaA domains comprising T69 and P374 with their counterparts in S. aureus, L. monocytogenes, and L. innocua. (B) lmo2537 gene diagram. Arrows indicate the orientation and approximate sizes of the open reading frames. Parenthetic numbers give the sizes (in base pairs) of the intergenic regions flanking lmo2537. upp, uracil phosphoribosyltransferase; lmo2537, UDP-GlcNAc 2-epimerase; atpI, ATP synthase subunit I. The dotted line flanked by black triangles below lmo2537 indicates the positions of the primers used in the RT-PCR analysis. (C) Tris-acetate-EDTA-agarose gel electrophoresis of transcripts amplified by RT-PCR. Numbers on the left correspond to the sizes (in kilobases) on the DNA ladder. Lanes: 1, PCR on control DNA; 2, RT-PCR plus RNA; 3, RT-PCR without RT.
The gene lmo2537 is flanked upstream by upp, encoding a putative uracil phosphoribosyltransferase, and downstream by atpI, the first gene of a putative ATP synthase operon (Fig. 1B). Two typical Rho-independent transcription terminator precede the upp gene and follow lmo2537, respectively. A canonical AGGAGG Shine-Dalgarno sequence precedes the lmo2537 coding sequence (7 bp upstream of the start codon). Notably, in B. subtilis, the upp gene is immediately followed by the ATP synthase operon, and mnaA is located in another region of the chromosome. We confirmed by RT-PCR (see Materials and Methods) that the lmo2537 gene was transcribed in the wild-type strain EGD-e grown in BHI medium at 37°C in exponential phase (Fig. 1C).
Construction of a conditional chromosomal deletion mutant of lmo2537.
We initially tried to construct a chromosomal knockout mutant of EGD-e (see Materials and Methods for details) by using a standard allelic replacement procedure (39). Chromosomal integration of the recombinant plasmid carrying the deleted region (pGts-A-Km-B) was obtained via a single crossover, yielding the merodiploid strain EGDpGΔlmo2537-lmo2537. All our attempts to obtain the second crossover, leading to plasmid excision and allelic exchange, were unsuccessful, suggesting that the deletion of lmo2537 was lethal.
To demonstrate this assumption, a conditional mutant was constructed. First, the wild-type gene lmo2537 was cloned under the IPTG-inducible SPAC promoter control, in the gram-negative/gram-positive shuttle plasmid pLIV-1 (15). The recombinant plasmid was then introduced into EGDpGΔlmo2537-lmo2537. After several cycles of growth at permissive temperature (30°C) in the presence of kanamycin, chloramphenicol, and 1 mM IPTG, allelic exchange of the chromosomal copy of lmo2537 occurred.
The conditional mutant (EGDΔlmo2537/pLiv-lmo2537) thus carries a chromosomal deletion of lmo2537 (substituted by a Kmr cassette) and a complementing wild-type lmo2537 allele under an IPTG-inducible promoter control.
Characteristics of the conditional mutant.
The role of lmo2537 in TA synthesis was first confirmed by determining the amounts of phosphate present in the cell wall TA of the conditional mutant and wild-type strains (Table 3). In the presence of IPTG, the value for the cell wall phosphate of the mutant strain was close to the value obtained with EGD-e (1.39 μmol of phosphate per mg of cell wall compared to 1.49 μmol of phosphate per mg of cell wall). However, in the IPTG-free medium, the amount of cell wall phosphate in the mutant strain was significantly reduced down to 0.77 μmol of PO4 per mg of cell wall. These observations are compatible with the involvement of lmo2537 in the synthesis of the linkage unit that is required for the synthesis of the main TA chain. The observed reduction (48%) in the cell wall phosphate is comparable to that (52%) of a B. subtilis thermosensitive mnaA-deficient strain at the nonpermissive temperature (58).
TABLE 3.
Effect of reduced lmo2537 expression on cell wall TA content
| Strain | Mean amt of cell wall phosphate (μmol/mg of cell wall) ± SDa
|
|
|---|---|---|
| Without IPTG | With 1 mM IPTG | |
| EGD-e | 1.49 ± 0.04 | ND |
| EGD-pLiv | 1.39 ± 0.02 | 1.42 ± 0.07 |
| EGDΔlmo2537/pLiv-lmo2537 | 0.77 ± 0.06 | 1.35 ± 0.04 |
Values are based on four independent measurements obtained on the same cell wall preparation.
We then monitored the growth of the conditional mutant in BHI medium supplemented with various concentrations of IPTG (from 0 to 1 mM IPTG), in liquid culture or on solid medium.
A control EGD-e strain, transformed with the pLIV plasmid without insert (denoted EGDpLIV), was constructed. In the absence of chloramphenicol, the growth of EGDpLIV was identical to that of EGD-e, and no significant loss of the plasmid was observed (not shown). In the presence of chloramphenicol, the growth of EGDpLIV was slightly reduced compared to that of EGD-e, most likely due to the translation-inhibitory effect of chloramphenicol. In the presence of 1 mM IPTG, the growth of the mutant strain was only slightly lower that of EGDpLIV, reflecting complementation of the chromosomal deletion by the wild-type allele under pSPAC control. In contrast, the growth of the mutant strain was reduced at lower IPTG concentrations (Fig. 2A). After 8.5 h, when the culture containing 1 mM IPTG had reached a plateau, the OD600 recorded in the culture without IPTG was twofold lower. Moreover, the mutant strain was unable to grow on BHI plates that did not contain IPTG (Fig. 2B), confirming the essential role of lmo2537 in bacterial survival.
FIG. 2.
Growth properties of the conditional mutant. (A) Growth kinetics in broth at 30°C (in BHI for EGD-e and in BHI-Cm supplemented with various concentrations of IPTG for EGDpLIV and EGDΔlmo2537/pLivlmo2537). (B) Growth at 30°C on BHI plates, supplemented with IPTG 1 mM (upper panel) or without IPTG (lower panel). In both cases, the bacteria were initially grown to stationary phase in liquid (in BHI for EGD-e [WT] and in BHI-Cm containing IPTG 1 mM for EGDΔlmo2537/pLivlmo2537 [Mut]). Mut, EGDΔlmo2537/pLivlmo2537.
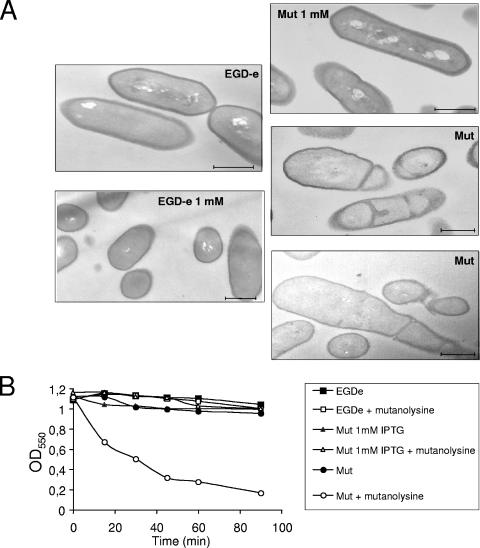
The morphology of the conditional mutant was compared to that of EGD-e by transmission electron microscopy. In thin sections (Fig. 3A), wild-type bacteria presented a typical morphology of dividing bacteria with formation of septum, a normal cell wall thickness, and a normal bacterial size (ca. 2 μm). When grown in the presence of 1 mM IPTG, the mutant strain had a normal morphology (Fig. 3A). In contrast, in the absence of IPTG, the conditional mutant exhibited a clearly altered morphology with an irregular cell wall thickness, and many bacteria were apparently arrested during cell division. Altogether, the data indicate that lmo2537 expression is required for both maintaining the shape of the bacterial envelope and for normal cell division.
FIG. 3.
(A) Transmission electron microscopy. The left panels show EGD-e grown in BHI with or without 1 mM IPTG; the right panels show the conditional mutant (Mut) grown in BHI with either 1 mM or no IPTG. (B) Sensitivity to mutanolysin. Bacteria were incubated with mutanolysin (50 U/ml [final concentration]) at 30°C for 90 min. At selected intervals, the OD550 of the suspensions was determined.
To assess the integrity of the cell wall, we tested the susceptibility of the conditional mutant to lysis after incubation with a cell wall hydrolase, mutanolysin. In the absence of IPTG induction, the conditional mutant appeared to be extremely sensitive to mutanolysin (Fig. 3B), whereas in the presence of IPTG it was as resistant as wild-type EGD-e. This assay demonstrated thus that reduced lmo2537 expression impaired cell wall integrity.
The susceptibility of the conditional mutant to a series of antibiotics was also evaluated on plates containing limiting amounts of IPTG (data not shown). The conditional mutant showed an increased sensitivity toward the peptidic antibiotics bacitracin, colistin, and polymyxin B. After 48 h of incubation at 30°C on a plate supplemented with 10 μM IPTG (the lowest concentration that still promoted homogeneous bacterial growth [see Materials and Methods]), the three disks were surrounded by clear halos of growth inhibition. The diameters of the halos were of 25, 10, and 9 mm with bacitracin, colistin, and polymyxin B, respectively. In contrast, no growth inhibition (colistin) or a turbid halo (bacitracin and polymyxin B) was observed around these disks on the plate supplemented with 1 mM IPTG (as well as with EGD-e). The sensitivity to other antibiotics such as clindamycin, pristinamycin, ofloxacin, rifampin, fosfomycin, minomycin, gentamicin, vancomycin, and teicoplonin was not altered.
We finally compared the protein content of envelope fractions or culture supernatants from the mutant strain to those of wild-type EGD-e. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses revealed that, for both fractions, the protein patterns were similar between the mutant irrespective of the presence of IPTG and the wild-type strain (data not shown). Thus, the reduction of Lmo2537 production did not result in global alterations of the protein composition of the cell envelope or to the nonspecific release of proteins in the culture supernatant.
Role of lmo2537 in intracellular survival and virulence.
The kinetics of intramacrophagic multiplication (Fig. 4A) of the conditional mutant was evaluated in the J774 cell line and compared to that of EGD-e. The conditional mutant was first grown overnight in BHI containing 1 mM IPTG, and infection of the cells was performed either in the presence (1 mM) or in the absence of IPTG. In the presence of IPTG in the cell culture medium, the intracellular survival and multiplication of the mutant was only slightly lower than that of EGD-e, reflecting efficient complementation of the lmo2537 activity in the cells. When infection was performed in the absence of IPTG, bacteria were able to grow up to 4 h after entry. The number of viable bacteria then dropped drastically (up to 10-fold, after two more hours).
FIG. 4.
Intracellular behavior of EGDΔlmo2537/pLivlmo2537. (A) The intracellular growth of the conditional mutant was compared to that of EGD-e in J774 macrophages. Invasion and intracellular multiplication was monitored in J774 macrophages at a bacterium/macrophage ratio of 10:1 over a 6-h period in the presence of gentamicin at 50 μg/ml (final concentration) as described previously (27). At selected intervals, infected J774 cells were lysed, and the titer of viable bacteria was determined by spreading cells onto BHI plates containing (in all cases) 1 mM IPTG. Mut+IPTG:cells+IPTG, EGDΔlmo2537/pLivlmo2537 was grown in BHI containing 1 mM, and infection was performed in the presence of 1 mM IPTG. Mut+IPTG:cells−IPTG, EGDΔlmo2537/pLivlmo2537 was grown in BHI containing 1 mM, and infection was performed in the absence of IPTG. Mut-IPTG:cells−IPTG, EGDΔlmo2537/pLivlmo2537 was grown in BHI containing 10 μM IPTG, and infection was performed in the absence of IPTG. (B) Fluorescence analyses. Infections of J774 macrophage-like cells were performed at a bacterium/macrophage ratio of 10:1. At 30 min (a) or 4 h (b) postinfection, J774 macrophages infected by EGD-e expressing GFP under plmo2537 promoter control were collected and processed for fluorescence analysis. (C) Real-time PCR. The induction ratio of lmo2537 transcription in EGD-e was quantified under two growth conditions: (i) in infected J774 macrophage-like cells after 30 min and 4 h of infection or (ii) in DMEM or BHI broth.
Notably, when the conditional mutant was grown overnight under low-IPTG (10 μM) conditions, with prior infection and intracellular survival monitored in the absence of IPTG, no bacterial multiplication was observed. The number of viable bacteria after 6 h was even slightly lower than upon entry. Under these conditions, microscopic examination did not reveal a major cytotoxic effect of bacterial growth.
After cell transfer of the conditional mutant from BHI medium containing 1 mM IPTG to IPTG-free medium (Fig. 2), the OD600 of the culture increased over 8 h. In contrast, the intracellular viable count, after an initial increase over 4 h, dropped significantly, suggesting that TA may contribute to the virulence of L. monocytogenes. However, it could not be ruled out that the TA content has a more significant impact on the viability of L. monocytogenes intracellularly than in broth culture.
Expression of lmo2537 during the intracellular life cycle.
We first used a GFP reporter construct to monitor the expression of the lmo2537 promoter region upon infection of the macrophage-like cell line J774 (Fig. 4B). The 260-bp fragment immediately preceding lmo2537 was inserted upstream of a promotorless gfp copy carried by the multicopy shuttle plasmid pAT28 (48), and the recombinant plasmid (pATgfp/plmo2537) was introduced into EGD-e. Bacteria were visualized with a confocal microscope by fluorescence and by negative contrast.
After 30 min of infection, all of the bacteria (approximately three per infected cell) were fluorescent (Fig.4Ba). After 4 h of infection, the bacteria had multiplied actively in the host cell cytosol and were also all fluorescent (Fig.4Bb). Our earlier confocal and electron microscopy observations with L. monocytogenes EGD-e have indicated that, after 30 min, a majority of bacteria are still localized inside phagosomes (54). In contrast, after 4 h of infection all of the bacteria are multiplying in the cytosol surrounded by polymerized actin (between 30 min and 4 h, the number of intracellular bacteria is approximately multiplied by 10 [results not shown]). Thus, the gfp reporter assay indicates that lmo2537 transcription is taking place in both phagosomal and cytosolic compartments.
Real-time RT-PCR was used to monitor quantitatively the transcription of lmo2537 in EGD-e upon infection of J774 macrophages and in BHI. This assay revealed comparable levels of lmo2537 expression after 30 min or 4 h of J774 infection (Fig. 4C), suggesting a constant expression throughout the intracellular life cycle. As a control, we verified that DMEM (the medium used for the cellular infection) was not responsible for this downregulation. Thus, gene lmo2537 appears to be transcribed both in broth and in infected cells.
Virulence and in vivo persistence of the conditional mutant.
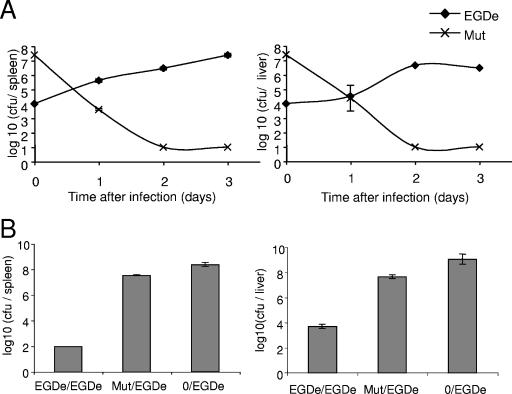
We first determined the LD50 of the conditional mutant in Swiss mice infected intravenously. The LD50 of the mutant was ∼10,000-fold higher than that of EGD-e. We then examined the in vivo persistence of EGDΔlmo2537/pLiv-lmo2537 mice (infected with nonlethal doses of 2 × 107 or 104 bacteria per mouse) versus EGD-e mice (infected with a sublethal dose of 104 bacteria per mouse) . As shown in Fig. 5A, EGDΔlmo2537/pLiv-lmo2537 had completely disappeared from infected spleens within 2 days after infection, whereas the lower inoculum of 104 EGD-e resulted in a peak of replication at day 3 after infection, reaching up to 107 bacteria per organ.
FIG. 5.
Kinetics of survival in infected organs and immunization assay. (A) The kinetics of bacterial growth were monitored in mice infected with either EGD-e (⧫) or the conditional mutant (×). Mice were inoculated intravenously with 0.5 ml of bacterial suspension (containing 104 bacteria for EGD-e or 2 × 107 bacteria for the conditional mutant). Bacterial survival in the spleen (left) and liver (right) was monitored over a 3-day period. Values and error bars represent, respectively, the means of the number (in log10) of bacteria per organ (five mice per point at day 1, 2, and 3) and the standard deviations for each point. (B) Protection. After immunization and challenge, the bacterial counts were determined in the spleen (left) and the liver (right) 3 days after the challenge. EGDe/EGDe, mice immunized with 104 EGD-e bacteria and challenged with 105 EGD-e; Mut/EGDe, mice immunized with 107 EGDΔlmo2537/pLivlmo2537 and challenged with 105 EGD-e; and 0/EGDe, nonimmunized mice control group challenged with 105 EGD-e.
Protection against challenge with wild-type L. monocytogenes.
To test for protection afforded by a preimmunization with EGDΔlmo2537/pLiv-lmo2537, mice were challenged after 28 days (see Materials and Methods for details) with 105 wild-type bacteria, and the bacterial loads in the spleens and livers were determined 3 days after challenge. Challenged naive mice had high levels of bacteria in their spleens at 3 days postinfection (>108; Fig. 5B). In contrast, EGD-e-immune mice had no splenic bacteria after challenge (<102; Fig. 5B). The mice that had received a single dose of EGDΔlmo2537/pLiv-lmo2537showed only a ∼10-fold reduction of bacterial numbers by day 3, suggesting a weak protective immunity.
DISCUSSION
We demonstrate in the present study that gene lmo2537, encoding a member of the UDP-GlcNAc 2-epimerase family, is an essential gene of L. monocytogenes. Since the function of this gene is to provide a precursor of the TA linkage unit that serves as an acceptor for the main TA chain assembly, our findings suggest that L. monocytogenes TAs are essential for maintaining the cell shape and viability. In vivo experiments demonstrated that TA expression is essential for the in vivo survival of L. monocytogenes.
Biological importance of TA in L. monocytogenes.
Most of the studies aimed at understanding the genetic organization, transcriptional regulation, and function of TA in gram-positive organisms have been performed in B. subtilis strain 168 (37). In conditions with added phosphate, B. subtilis 168 cell walls were shown to contain two types of TA: poly(glycerol phosphate), which is essential for cell growth, and nonessential poly(glucosyl N-acetylgalactosamine 1-phosphate), which is attached to the peptidoglycan most likely via a common linkage unit (23). Mutations in all of the tag genes required for poly(glycerol phosphate) synthesis result in a reduction of the cell wall phosphate content and in considerable changes in cell shape (see reference 58 and references therein). Of interest, when B. subtilis is grown under phosphate-limited conditions, the TAs are replaced by teichuronic acid (18). This represents an important mechanism for conserving phosphate since the anionic polymer composition of the cell wall changes from phosphate-containing TA to phosphate-free teichuronic acids. Teichuronic acid synthesis is governed by the tua operon (tuaABCDEFGH) (60). By computer-assisted analysis of the genome of EGD-e, we did not identify any orthologous tua operon (using the genome comparison facility of the TIGR Center, available at the internet address http://www.tigr.org/tigr-scripts/CMR2/GenomesRegionComparisonForm.dbi?db). L. monocytogenes, which expresses only TA, must have developed regulatory mechanisms to reduce TA expression under conditions of phosphate starvation and, in particular, under the phosphate-limiting conditions encountered during its infectious cycle. The ionic composition of the eukaryotic cytosol has not been precisely characterized (for a review, see reference 3). Most of the information comes from indirect studies on the regulation of bacterial genomes during intracellular multiplication. Notably, a recent study on the transcriptional adaptation of Shigella flexneri during infection of eukaryotic cells (40) revealed that the phoRB regulon, responsible for phosphate uptake in this organism, was significantly upregulated during intracytosolic growth, suggesting that the cytosol has restricted levels of available phosphate.
The slightly reduced expression of lmo2537 measured by real-time PCR is consistent with this hypothesis and might reflect the overall reduction of TA expression by intracellular bacteria. In addition, the fact that lmo2537 is an essential gene suggests that a minimal amount of TA expression must be achieved to allow cell shape maintenance and cell division (7) in infected host cells.
The cell wall of most gram-positive bacteria contains also lipoteichoic acid (LTA), an anionic polymer anchored to the membrane by a glycolipid moiety (20). Both TA and LTA confer a high density of negative charge pathways (47) to the bacterial surface. Although TA and LTA are assembled via different pathways (see reference 47 and references therein), both possess ester-linked d-alanine residues. The degree of d-alanylation in LTA and TA can vary considerably between species and in the function of the growth medium. Such variations modulate the net negative charge of the bacterial surface. Strikingly, although d-alanylation is not essential for bacterial viability, it participates in virulence in several pathogenic bacterial species (1, 14, 49, 52). In particular, in L. monocytogenes, the degree of alanylation of TA, which varies between species, has been demonstrated to contribute to class IIa bacteriocin resistance (62).
Reduced expression of lmo2537 reduced the amount of TA in the cell wall. This led to an increased sensitivity toward the peptidic antibiotics bacitracin, colistin, and polymyxin B, as well as to an increased sensitivity to the cell wall hydrolase mutanolysin. Notably, the lack of d-alanylation of LTA and TA, in a dltA mutant of L. monocytogenes, has been previously shown (1) to increase sensitivity to colistin and polymyxin B, whereas sensitivity to bacitracin was not affected. Since TA is covalently attached to peptidoglycan, under conditions of reduced TA production, the bacteria are likely to be more sensitive to drugs affecting the cell wall (bacitracin inhibits regeneration of the membrane lipid carrier involved in peptidoglycan biosynthesis, whereas polymyxin and colistin disrupt the phospholipid layer in cell membranes).
The gene lmo2537 is downregulated in eukaryotic cells.
The lmo2537 gene was initially identified as an in vivo-inducible locus. The present data revealed that the expression of lmo2537 is fourfold lower during intracellular multiplication than during growth in broth and remains rather constant throughout the intracellular life cycle of the bacteria. This apparent discrepancy can be explained by the lack of quantification in the IVET approach. Indeed, the ivi loci had been identified by screening genomic libraries of L. monocytogenes chromosomal DNA on the basis of a poor to undetectable LLO (the pore-forming cytolysin secreted by L. monocytogenes) activity (as determined on blood agar plates) and sufficient LLO expression to restore virulence in the mouse (16). It is, therefore, likely that the low lmo2537 expression in cells is sufficient to produce enough LLO to promote phagosomal escape and to restore virulence.
Lmo2537, a new target for antibacterial chemotherapy?
The emergence of antibiotic resistance among important bacterial pathogens has generated the need to develop new and more effective therapies. Several experimental approaches have been developed very recently for the large-scale identification of essential genes in a number of bacterial species. The systematic disruption of open reading frames has been undertaken either by transposon insertion tests or by plasmid insertion mutagenesis. This second strategy works best with naturally competent organisms, such as Streptococcus pneumoniae (69), and Bacillus subtilis (36). A similar approach based on insertion-duplication mutagenesis was recently described to screen for essential genes of Salmonella enterica serovar Typhimurium by using a bank of genomic fragments cloned into a conditionally replicating vector (35).
Alternatively, a shotgun antisense RNA method has been applied to identify conserved genes in S. aureus (21). Finally, computational approaches have also been developed. A list of all currently available essential genes has been compiled (70) into a database (DEG [http://tubic.tju.edu.cn/deg/]) that includes the essential genes identified in nine bacterial genomes. Such strategies have not yet been undertaken in L. monocytogenes. To date, lmo2537 represents the first experimentally demonstrated essential gene. The UDP-GlcNAc 2-epimerase activity encoded by lmo2537 is conserved among many gram-positive bacterial pathogens, including Bacillus anthracis, Streptococcus pneumoniae, Staphylococcus aureus, and Clostridium perfringens, and could thus constitute an interesting target for the development of broad-range antibacterial chemotherapy.
Conditional mutants: possible candidates for a new generation of live bacterial vaccines?
Recently, an L. monocytogenes live vaccine candidate has been proposed based on the conditional complementation of an amino acid deficiency. An attenuated strain of L. monocytogenes carrying chromosomal deletion of two genes (dal and dat) used for d-alanine synthesis was supplemented in trans by a suicide plasmid expressing the dal and dat genes, allowing transient d-alanine synthesis. The recombinant strain generated efficient cellular immune response and afforded full protection against lethal challenge by wild-type L. monocytogenes (71), prompting us to evaluate the potential vaccine efficacy of our mutant in the mouse model. The conditional mutant appeared to be rapidly eliminated from infected animals and did not allow, in the conditions used, the induction of an efficient protection. However, it is possible that other conditions, such as repeated priming, higher doses of bacteria, or transient feeding mice with IPTG (68), would increase in vivo bacterial persistence and thus lead to full protection. The conditional expression of essential genes might allow the development of totally safe live recombinant bacterial vaccines.
Acknowledgments
This study was supported by CNRS, INSERM, University Paris V, and by grant 3100A0-102205 (to V.L. and B.S.) from the Swiss National Science Foundation.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate, and phosphatases. Methods Enzymol. 8:115-118. [Google Scholar]
- 3.Appelberg, R. 2006. Macrophage nutriprive antimicrobial mechanisms. J. Leukoc. Biol. [DOI] [PubMed]
- 4.Autret, N., and A. Charbit. 2005. Lessons from signature-tagged mutagenesis on the infectious mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 29:703-717. [DOI] [PubMed] [Google Scholar]
- 5.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autret, N., C. Raynaud, I. Dubail, P. Berche, and A. Charbit. 2003. Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence. Infect. Immun. 71:4463-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhavsar, A. P., L. K. Erdman, J. W. Schertzer, and E. D. Brown. 2004. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J. Bacteriol. 186:7865-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnemain, C., C. Raynaud, H. Reglier-Poupet, I. Dubail, C. Frehel, M. A. Lety, P. Berche, and A. Charbit. 2004. Differential roles of multiple signal peptidases in the virulence of Listeria monocytogenes. Mol. Microbiol. 51:1251-1266. [DOI] [PubMed] [Google Scholar]
- 10.Brehm, K., J. Kreft, M. T. Ripio, and J. A. Vazquez-Boland. 1996. Regulation of virulence gene expression in pathogenic Listeria. Microbiologia 12:219-236. [PubMed] [Google Scholar]
- 11.Bubert, A., Z. Sokolovic, S. K. Chun, L. Papatheodorou, A. Simm, and W. Goebel. 1999. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol. Gen. Genet. 261:323-336. [DOI] [PubMed] [Google Scholar]
- 12.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 13.Chen, P. S. 1956. Microdetermination of phosphorus. Anal. Chem. 18:1756-1758. [Google Scholar]
- 14.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. Van Kessel, J. A. Van Strijp, F. Gotz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 15.Dancz, C. E., A. Haraga, D. A. Portnoy, and D. E. Higgins. 2002. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J. Bacteriol. 184:5935-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubail, I., P. Berche, and A. Charbit. 2000. Listeriolysin O as a reporter to identify constitutive and in vivo-inducible promoters in the pathogen Listeria monocytogenes. Infect. Immun. 68:3242-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587-610. [DOI] [PubMed] [Google Scholar]
- 18.Ellwood, D. C., and D. W. Tempest. 1969. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem. J. 111:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler, F. 1988. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection 16(Suppl. 2):S92-S97. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, W., T. Mannsfeld, and G. Hagen. 1990. On the basic structure of poly (glycerophosphate) lipoteichoic acids. Biochem. Cell. Biol. 68:33-43. [DOI] [PubMed] [Google Scholar]
- 21.Forsyth, R. A., R. J. Haselbeck, K. L. Ohlsen, R. T. Yamamoto, H. Xu, J. D. Trawick, D. Wall, L. Wang, V. Brown-Driver, J. M. Froelich, G. C. Kedar, P. King, M. McCarthy, C. Malone, B. Misiner, D. Robbins, Z. Tan, Z. Y. Zhu Zy, G. Carr, D. A. Mosca, C. Zamudio, J. G. Foulkes, and J. W. Zyskind. 2002. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol. Microbiol. 43:1387-1400. [DOI] [PubMed] [Google Scholar]
- 22.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freymond, P. P., V. Lazarevic, B. Soldo, and D. Karamata. 2006. Poly(glucosyl-N-acetylgalactosamine 1-phosphate), a wall teichoic acid of Bacillus subtilis 168: its biosynthetic pathway and mode of attachment to peptidoglycan. Microbiology 152:1709-1718. [DOI] [PubMed] [Google Scholar]
- 24.Fujii, H., K. Kamisango, M. Nagaoka, K. Uchikawa, I. Sekikawa, K. Yamamoto, and I. Azuma. 1985. Structural study on teichoic acids of Listeria monocytogenes types 4a and 4d. J. Biochem. 97:883-891. [DOI] [PubMed] [Google Scholar]
- 25.Gahan, C. G., and C. Hill. 2000. The use of listeriolysin to identify in vivo induced genes in the gram-positive intracellular pathogen Listeria monocytogenes. Mol. Microbiol. 36:498-507. [DOI] [PubMed] [Google Scholar]
- 26.Glaser, P., L. Frangeul, C. Buchrieser, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durand, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. Mata Vicente, E. Ng, G. Nordsiek, S. Novella, B. de Pablos, J. C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, C. Rusniok, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 27.Glomski, I. J., M. M. Gedde, A. W. Tsang, J. A. Swanson, and D. A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harder, A., R. Wildgruber, A. Nawrocki, S. J. Fey, P. M. Larsen, and A. Gorg. 1999. Comparison of yeast cell protein solubilization procedures for two-dimensional electrophoresis. Electrophoresis 20:826-829. [DOI] [PubMed] [Google Scholar]
- 29.Jones, S., and D. A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamisango, K., H. Fujii, H. Okumura, I. Saiki, Y. Araki, Y. Yamamura, and I. Azuma. 1983. Structural and immunochemical studies of teichoic acid of Listeria monocytogenes. J. Biochem. 93:1401-1409. [DOI] [PubMed] [Google Scholar]
- 31.Kaya, S., Y. Araki, and E. Ito. 1985. Characterization of a novel linkage unit between ribitol teichoic acid and peptidoglycan in Listeria monocytogenes cell walls. Eur. J. Biochem. 146:517-522. [DOI] [PubMed] [Google Scholar]
- 32.Kaya, S., K. Yokoyama, Y. Araki, and E. Ito. 1984. N-acetylmannosaminyl(1→4)N-acetylglucosamine, a linkage unit between glycerol teichoic acid and peptidoglycan in cell walls of several Bacillus strains. J. Bacteriol. 158:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiser, K. B., N. Bhasin, L. Deng, and J. C. Lee. 1999. Staphylococcus aureus cap5P encodes a UDP-N-acetylglucosamine 2-epimerase with functional redundancy. J. Bacteriol. 181:4818-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klarsfeld, A. D., P. L. Goossens, and P. Cossart. 1994. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol. Microbiol. 13:585-597. [DOI] [PubMed] [Google Scholar]
- 35.Knuth, K., H. Niesalla, C. J. Hueck, and T. M. Fuchs. 2004. Large-scale identification of essential Salmonella genes by trapping lethal insertions. Mol. Microbiol. 51:1729-1744. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarevic, V., F. X. Abellan, S. B. Moller, D. Karamata, and C. Mauel. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148:815-824. [DOI] [PubMed] [Google Scholar]
- 38.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345-355. [DOI] [PubMed] [Google Scholar]
- 39.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucchini, S., H. Liu, Q. Jin, J. C. Hinton, and J. Yu. 2005. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect. Immun. 73:88-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahan, M. J., J. W. Tobias, J. M. Slauch, P. C. Hanna, R. J. Collier, and J. J. Mekalanos. 1995. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc. Natl. Acad. Sci. USA 92:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauck, J., and L. Glaser. 1972. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J. Biol. Chem. 247:1180-1187. [PubMed] [Google Scholar]
- 43.Mauel, C., M. Young, and D. Karamata. 1991. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J. Gen. Microbiol. 137:929-941. [DOI] [PubMed] [Google Scholar]
- 44.Miesel, L., J. Greene, and T. A. Black. 2003. Genetic strategies for antibacterial drug discovery. Nat. Rev. Genet. 4:442-456. [DOI] [PubMed] [Google Scholar]
- 45.Moors, M. A., B. Levitt, P. Youngman, and D. A. Portnoy. 1999. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect. Immun. 67:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair, S., C. Frehel, L. Nguyen, V. Escuyer, and P. Berche. 1999. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol. Microbiol. 31:185-196. [DOI] [PubMed] [Google Scholar]
- 47.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton, S. M., P. E. Klebba, C. Raynaud, Y. Shao, X. Jiang, I. Dubail, C. Archer, C. Frehel, and A. Charbit. 2005. The svpA-srtB locus of Listeria monocytogenes: fur-mediated iron regulation and effect on virulence. Mol. Microbiol. 55:927-940. [DOI] [PubMed] [Google Scholar]
- 49.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 50.Piuri, M., C. Sanchez-Rivas, and S. M. Ruzal. 2005. Cell wall modifications during osmotic stress in Lactobacillus casei. J. Appl. Microbiol. 98:84-95. [DOI] [PubMed] [Google Scholar]
- 51.Pooley, H. M., and D. Karamata. 2000. Incorporation of [2-3H]glycerol into cell surface components of Bacillus subtilis 168 and thermosensitive mutants affected in wall teichoic acid synthesis: effect of tunicamycin. Microbiology 146(Pt. 4):797-805. [DOI] [PubMed] [Google Scholar]
- 52.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 53.Rediers, H., P. B. Rainey, J. Vanderleyden, and R. De Mot. 2005. Unraveling the secret lives of bacteria: use of in vivo expression technology and differential fluorescence induction promoter traps as tools for exploring niche-specific gene expression. Microbiol. Mol. Biol. Rev. 69:217-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reglier-Poupet, H., C. Frehel, I. Dubail, J. L. Beretti, P. Berche, A. Charbit, and C. Raynaud. 2003. Maturation of lipoproteins by type II signal peptidase is required for phagosomal escape of Listeria monocytogenes. J. Biol. Chem. 278:49469-49477. [DOI] [PubMed] [Google Scholar]
- 55.Roth, Z. 1961. A graphic probit method for the calculation of LD50 and relative toxicity. Cesk. Fysiol. 10:408-422. (In Czech.) [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Expression of cloned genes in Escherichia coli, p. 17.37-17.41. In C. Nolan (ed.), Molecular cloning: a laboratory manual, vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 57.Slutsker, L., and A. Schuchat. 1999. Subtyping Listeria monocytogenes. Marcel Dekker, Inc., New York, N.Y.
- 58.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079-2087. [DOI] [PubMed] [Google Scholar]
- 59.Soldo, B., V. Lazarevic, P. Margot, and D. Karamata. 1993. Sequencing and analysis of the divergon comprising gtaB, the structural gene of UDP-glucose pyrophosphorylase of Bacillus subtilis 168. J. Gen. Microbiol. 139:3185-3195. [DOI] [PubMed] [Google Scholar]
- 60.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 61.Soldo, B., V. Lazarevic, H. M. Pooley, and D. Karamata. 2002. Characterization of a Bacillus subtilis thermosensitive teichoic acid-deficient mutant: gene mnaA (yvyH) encodes the UDP-N-acetylglucosamine 2-epimerase. J. Bacteriol. 184:4316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vadyvaloo, V., S. Arous, A. Gravesen, Y. Hechard, R. Chauhan-Haubrock, J. W. Hastings, and M. Rautenbach. 2004. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology 150:3025-3033. [DOI] [PubMed] [Google Scholar]
- 63.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, and B. Gonzalez-Zorn. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 65.Weidenmaier, C., A. Peschel, Y. Q. Xiong, S. A. Kristian, K. Dietz, M. R. Yeaman, and A. S. Bayer. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191:1771-1777. [DOI] [PubMed] [Google Scholar]
- 66.Wendlinger, G., M. J. Loessner, and S. Scherer. 1996. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142(Pt. 4):985-992. [DOI] [PubMed] [Google Scholar]
- 67.Wilson, R. L., A. R. Tvinnereim, B. D. Jones, and J. T. Harty. 2001. Identification of Listeria monocytogenes in vivo-induced genes by fluorescence-activated cell sorting. Infect. Immun. 69:5016-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu, J. D., H. C. Hsueh, W. T. Huang, H. S. Liu, H. W. Leung, Y. R. Ho, M. T. Lin, and M. D. Lai. 1997. The inducible lactose operator-repressor system is functional in the whole animal. DNA Cell Biol. 16:17-22. [DOI] [PubMed] [Google Scholar]
- 69.Zalacain, M., S. Biswas, K. A. Ingraham, J. Ambrad, A. Bryant, A. F. Chalker, S. Iordanescu, J. Fan, F. Fan, R. D. Lunsford, K. O'Dwyer, L. M. Palmer, C. So, D. Sylvester, C. Volker, P. Warren, D. McDevitt, J. R. Brown, D. J. Holmes, and M. K. Burnham. 2003. A global approach to identify novel broad-spectrum antibacterial targets among proteins of unknown function. J. Mol. Microbiol. Biotechnol. 6:109-126. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, R., H. Y. Ou, and C. T. Zhang. 2004. DEG: a database of essential genes. Nucleic Acids Res. 32:271-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao, X., Z. Li, B. Gu, and F. R. Frankel. 2005. Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product. Infect. Immun. 73:5789-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]