Abstract
A zinc ion-sensitive mutant of Mycobacterium smegmatis was isolated. The transposon insertion was located in zitA (MSMEG0750), a gene coding for a cation diffusion facilitator family protein. Zinc ions specifically induced expression of zitA. In silico analysis revealed that environmental and opportunistic pathogenic species contain higher numbers of cation diffusion facilitator genes than do obligate pathogens.
Zinc is an essential trace element. However, a high concentration of zinc ions causes toxicity. Therefore, cells require homeostasis mechanisms to control the intracellular zinc ion concentration. In bacteria, the zinc ion concentration is controlled by the activity of membrane transporters belonging to different families that mediate its uptake and efflux. The cation diffusion facilitators (CDFs) are integral membrane proteins that transport various divalent cations, including Zn(II) (19). Some of the CDF proteins have been shown to be specific for a single metal ion substrate whereas others possess broad substrate specificity (8, 15).
The genus Mycobacterium encompasses various environmental and pathogenic species. Zinc acts as an important component of many mycobacterial enzymes such as superoxide dismutase, alcohol dehydrogenase, carbonic anhydrase, etc. (5, 7, 25). Sufficient acquisition of zinc ions may therefore be important for production of zinc-containing enzymes in active form. Moreover, both pathogenic and environmental mycobacteria survive in various inhospitable conditions in external habitats (6, 12, 16). Resistance mechanisms become important for survival and growth in zinc ion-contaminated habitats. Studies aiming to understand zinc ion homeostasis mechanisms in mycobacteria have recently been initiated. Two transcriptional regulators shown to be induced by zinc ions have been identified from Mycobacterium tuberculosis and Mycobacterium smegmatis (13), and their role in regulation of genes involved in zinc ion homeostasis was later suggested (3). However, molecular determinants for zinc ion acquisition and resistance in mycobacteria are yet to be identified. Here we report the identification and characterization of a chromosomal zinc ion resistance determinant of M. smegmatis.
Isolation of a zinc ion-sensitive mutant.
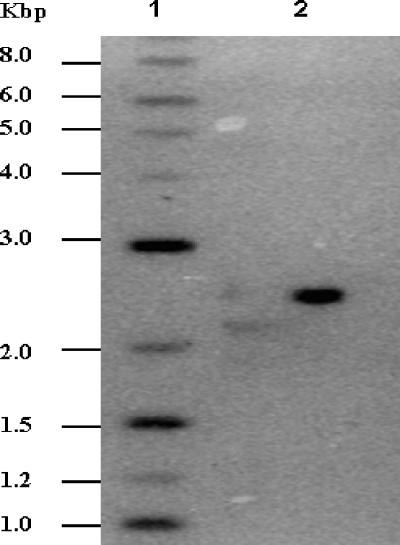
Strains, plasmids, and oligonucleotides used in this study are summarized in Table 1. A transposon mutant library of M. smegmatis mc2155 was prepared using a previously described protocol with minor modifications (22). The plasmid pMycoMar was transformed by electroporation (23), and the transformants were allowed to grow at 28°C for 24 h without antibiotic selection. The mutants were selected at 42°C on Luria-Bertani (LB) agar plates supplemented with 5% sucrose and kanamycin (20). The mutant library, consisting of more than 2,500 mutants, was screened for zinc ion sensitivity, and mutant K516, which failed to grow on 400 μM zinc acetate-containing LB agar, was identified. The MIC assays were performed by growing M. smegmatis strains for 24 h in LB broth with 0.05% Tween 80 and further inoculating the cultures as 1:200 dilutions in fresh medium containing a range of concentrations of different metal salts. The cell growth was monitored after 48 h as absorbance at 600 nm. Mutant K516 showed 10-fold-increased sensitivity to zinc ions in comparison to wild-type M. smegmatis mc2155; however, the sensitivity to other tested divalent metal ions remained unchanged (Table 2). Southern hybridization using an NdeI-BamHI fragment carrying the mariner minitransposon from pMycoMar as a probe revealed a single transposon insertion in the mutant chromosome (Fig. 1), indicating that the disrupted locus is responsible for the zinc ion-sensitive phenotype of the mutant.
TABLE 1.
Bacterial strains, plasmids and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Relevant characteristic(s) or sequence | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F−mcrA Δ(mrr-hsdRMSmcrBC) φ80dlacZ ΔM15 ΔlacX74 deoR recA1 araΔ139 (ara leu)7697 galU galK λ−rpsL endA1 nupG | Gibco-BRL |
| M. smegmatis mc2155 | High-frequency transformation strain derived from wild-type M. smegmatis mc2-6 | 23 |
| M. smegmatis LR222 | High-frequency transformation strain derived from wild-type M. smegmatis mc2-6 | 23 |
| K516 | M. smegmatis mc2155; zitA::Tn Kmr | This work |
| Plasmids | ||
| pUC19 | Sequencing vector; lacZα Apr | MBI Fermentas |
| pSMT3 | E. coli-Mycobacterium shuttle vector; Hygr | 10 |
| pSMT3*a | E. coli-Mycobacterium shuttle vector; hsp60 promoter; Hygr | 17 |
| pJEM13 | E. coli-Mycobacterium promoter-probe shuttle vector for lacZ fusions; Kmr | 24 |
| pAG1 | M. smegmatis zitA with native promoter cloned in pSMT3 | This work |
| pAG2 | zitA promoter cloned in pJEM13 | This work |
| pAG3 | MAP3865c gene cloned in pSMT3* | This work |
| pAG4 | MAP2784 gene cloned in pSMT3* | This work |
| pAG5 | Rv2025c gene cloned in pSMT3* | This work |
| pAG6 | ML0283 gene cloned in pSMT3* | This work |
| pAG7 | MSMEG0750 gene (zitA) cloned in pSMT3* | This work |
| Oligonucleotides | ||
| MarA | TAG CGA CGC CAT CTA TGT GTC | 21 |
| MarB | CTT GAA GGG AAC TAT GTT G | 21 |
| ZitAF | CCC AAG CTT AGG TCA GCC GCG CGA CGT GCA CCG | This work |
| ZitAR | TGC TCT AGA TCA CTC GGC CTC GCA CCT GCA GTC | This work |
| ZitApF | TAC GGT ACC ATC CGC AGG CGT GCG ATT CCG TGC | This work |
| ZitApR | CTT GGT ACC CAT CCG GCT CAC CCG TGC GTC GGT | This work |
| MSMEG0750 5′ | ATC AAG CTT AAT GGG CGC GGG CCA CGA TCA CAG | This work |
| MSMEG0750 3′ | GAT AAG CTT TCA CTC GGC CTC GCA CCT GCA GTC | This work |
| MAP3865 5′ | GGC TGC AGG ATG GGC GCC GGC CAC AAC CAC ACC | This work |
| MAP3865 3′ | GAT AAG CTT TCA GAA GCT GTC GGA GCA CTC GG | This work |
| MAP2784 5′ | GGC TGC AGG ATG CAC GTG ATC AAC GGG GTC CGC | This work |
| MAP2784 3′ | GAT AAG CTT TCA TCT GGC AGG GAT CGC TGA TCG | This work |
| Rv2025c 5′ | GGC TGC AGG ATG ACC CAC GAC CAC GCT CAT TC | This work |
| Rv2025c 3′ | GAT AAG CTT TCA CTC TAC GGT GCG GCC ACG ATC | This work |
| ML0283 5′ | GGC TGC AGG TTG CAA TGC ATG CGT AAA CAC GC | This work |
| ML0283 3′ | GAT AAG CTT TCA GGT GCG CGG TGG GAC ATA TC | This work |
Because two plasmids used in this study have the same name, the one with the hsp60 promoter is identified with an asterisk.
TABLE 2.
MICs of different metal salts
| Metal salt | Apparent MIC (mM)a
|
|
|---|---|---|
| M. smegmatis mc2155 | K516 (zitA::Tn) | |
| Zn(OAc)2 | 1.500 | 0.150 |
| Cd(OAc)2 | 0.015 | 0.015 |
| CoCl2 | 1.250 | 1.250 |
| NiCl2 | 2.000 | 2.000 |
| CuSO4 | 4.000 | 4.000 |
| MnCl2 | 5.000 | 5.000 |
The apparent MIC is the concentration of metal ion at which no cell growth was observed.
FIG. 1.
Southern blot analysis of zinc ion-sensitive mutant K516. The genomic DNA from M. smegmatis mutants was isolated and digested with BamHI, an enzyme that does not cut within the transposon. The blot was probed with a purified NdeI-BamHI fragment carrying the mariner minitransposon. Lane 1, 2-log DNA ladder; lane 2, mutant K516.
Mapping of transposon insertion site and sequence analysis.
The transposon- and flanking-region-containing fragment was cloned after BamHI digestion of genomic DNA from the mutant and used for determining the DNA sequence flanking the transposon using primers MarA for the 5′ insertion site and MarB for the 3′ insertion site (21). The sequences obtained were searched using the Basic Local Alignment Search Tool (BLAST) in M. smegmatis genome sequence databases (http://tigrblast.tigr.org/cmr-blast/) to map the transposon insertion site. The transposon insertion was located 462 bp downstream of the translation initiation site of an open reading frame predicted to encode a putative cobalt-zinc-cadmium resistance protein (TIGR accession no. MSMEG0750). Since the disruption of this gene resulted in zinc ion sensitivity of the mutant, it was designated zitA (zinc ion tolerance gene A).
ZitA shared 40% identity with the cobalt, zinc, and cadmium resistance protein CzcD of Cupriavidus (formerly Ralstonia) metallidurans CH34 (2); 36% identity with the zinc resistance protein ZitB of Escherichia coli (8); and 29% identity with the zinc and cobalt resistance protein ZntA of Staphylococcus aureus RN450 (26). ZitA has one histidine-rich region at the amino terminus (four amino acids out of six), which may be involved in zinc ion binding. Similar histidine-rich regions have been predicted for binding of metal ions in ZntA and ZitB (11, 26). In contrast to two other characterized bacterial zinc ion-transporting CDF homologues, ZitB and ZntA, ZitA has no histidine-rich region at the carboxy terminus. It was similar to CzcD, which also lacks a histidine-rich region at the carboxy terminus. An amino acid sequence alignment of ZitA with characterized bacterial CDF proteins, i.e., CzcD, ZitB, and ZntA (data not shown), revealed that the majority of the conserved residues found important for activity of ZitB and CzcD were present in ZitA (except H159 of ZitB and C290 and H298 of CzcD), indicating that these proteins use similar mechanisms for zinc ion detoxification (1, 11).
zitA complements the zinc ion-sensitive mutant.
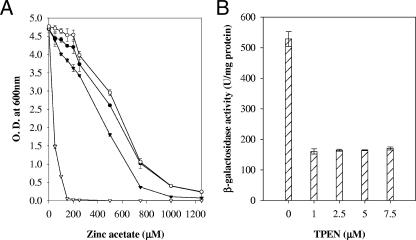
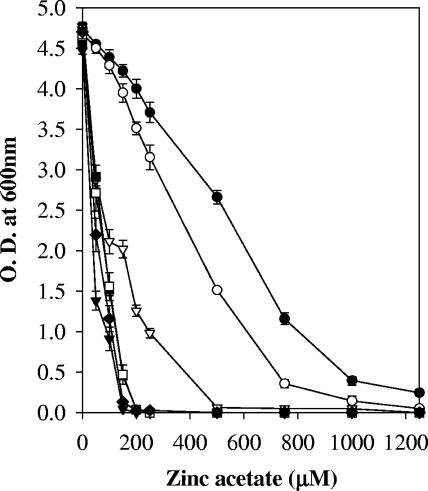
The zitA gene along with the putative promoter region was PCR amplified using primers ZitAF and ZitAR and cloned in pSMT3 to create pAG1. Mutant K516 and M. smegmatis mc2155 were transformed with pAG1 and vector control. The MICs of zinc ion were 1,250 μM and 150 μM for K516/pAG1 and K516/pSMT3, respectively. Thus, expression of zitA from a multicopy plasmid, pAG1, was able to complement the zinc ion sensitivity of the mutant (Fig. 2A). Disruption of zitB, a close homologue of zitA in E. coli, did not affect zinc ion sensitivity, and only a double mutant, zitB along with zntA (a P-type ATPase), exhibited hypersensitivity to zinc ions (8), confirming that zitB is an additional determinant and that zntA acts as a major determinant for zinc ion resistance in E. coli, whereas disruption of zntA, a CDF, from S. aureus resulted in 10- and 6-fold sensitivity to zinc and cobalt ion, respectively (26). Our results showed that disruption of zitA leads to zinc ion sensitivity of the mutant to a greater extent, while sensitivity to other metal ions remained unaffected, indicating that zitA is a major and specific determinant of zinc ion resistance in M. smegmatis.
FIG. 2.
(A) Effect of zinc ion on growth of M. smegmatis mc2155/pAG1 (○), M. smegmatis mc2155/pSMT3 (•), K516 (zitA::Tn)/pAG1 (▾), and K516 (zitA::Tn)/pSMT3 (▿). The graph depicts growth in the presence of different concentrations of zinc acetate. Experiments were performed in triplicate; values are averages, and standard deviations are shown as error bars. (B) Repression of zitA expression by TPEN. The cultures were grown in the presence of variable concentrations of the metal ion chelator TPEN in Sauton medium. β-Galactosidase activity was monitored and expressed as nanomoles of o-nitrophenol produced min−1 mg of protein−1. Experiments were performed in triplicate; values are averages, and standard deviations are shown as error bars.
zitA is induced by zinc ions.
To analyze metal-dependent expression of zitA, a translational fusion using lacZ as a reporter gene was constructed. The promoter sequence was searched by using the prokaryotic option of the neural network promoter prediction algorithm of the Berkeley Drosophila Genome Project (http://www.fruitfly.org/seq_tools/promoter.html). A 550-bp region containing the putative promoter, ribosomal binding site, and translation initiation site along with 18 initial codons of the zitA gene was amplified by using primers ZitApF and ZitApR by PCR. The PCR product was cloned in promoter-probe vector pJEM13 in the KpnI restriction site (24). The construct with correct orientation was selected by restriction digestion. The resultant plasmids pAG2 and pJEM13 were electroporated in M. smegmatis mc2155. The cultures from mid-log phase were diluted in fresh Sauton medium and were grown at 37°C to an optical density at 600 nm of 1.0. Induction with different metal ions was carried out for 6 h. A zinc ion chelator, TPEN [tetra-kis-(2-pyridylmethyl)ethylenediamine; Sigma], was added in culture media as needed. Cells were then disrupted by sonication and centrifuged at 12,000 × g for 10 min at 4°C. β-Galactosidase activity of the extracts was measured as previously described (14). Total protein was determined using a protein determination kit (BCA-1 kit; Sigma) according to the supplier's instructions. The enzyme activity was expressed as nanomoles of o-nitrophenol produced min−1 mg of protein−1. Background β-galactosidase activities were estimated using vector control in each experiment and were subtracted from the tests. The background activities were always less than 10% of the activities obtained for the test. The β-galactosidase activity of M. smegmatis mc2155/pAG2 grown in Sauton medium or Chelex-100 (10 g/liter)-treated Sauton medium, supplemented with filter-sterilized MgSO4 and ferric ammonium citrate solution (13), was 585.71 ± 30.57 U/mg protein, which indicated a possibility of zinc ion contamination in Sauton medium even after Chelex-100 treatment. Reporter assays were then performed with cultures grown in Sauton medium containing variable concentrations of the zinc ion chelator TPEN (4, 18). TPEN addition to culture medium at a final concentration of 1.0 μM was able to reduce promoter activity about threefold, whereas further additions of TPEN of up to 7.5 μM did not reduce expression of the gene below a basal β-galactosidase activity of 170.10 ± 5.30 U/mg protein (Fig. 2B).
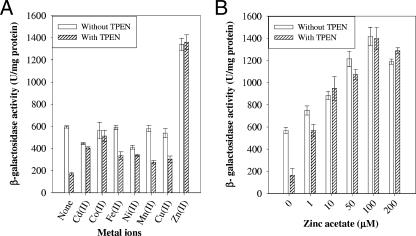
To examine the metal ion specificity of zitA expression, β-galactosidase assays were performed with cultures grown in medium supplemented with TPEN or not. Cultures were induced with different metal ions at 100 μM (except for cadmium salt, which was used at 10 μM) for 6 h and then used for β-galactosidase assays. Zinc was able to induce promoter activity about twofold in comparison to control (Fig. 3A). In 1.0 μM TPEN-supplemented Sauton medium, addition of zinc ions induced β-galactosidase activity more than sixfold in comparison to the respective control. In contrast, other metal ions did not induce expression from the zitA promoter. Addition of other metal ions to TPEN-supplemented Sauton medium increased β-galactosidase activity approximately close to the level in the control without TPEN. This is expected due to competitive release of TPEN-bound zinc ions by the presence of a manyfold-higher concentration of other metal ions. The concentration dependence of zitA expression by zinc ions was examined. Induction was observed with 1.0 μM zinc acetate and reached a maximum at 100 μM (Fig. 3B). These results imply that zitA is specifically regulated by zinc ion in the micromolar range.
FIG. 3.
Induction of zitA. M. smegmatis mc2155 cultures were grown in Sauton medium supplemented with 1 μM TPEN or not. (A) Metal-dependent regulation of zitA expression. Induction with different metal ions was done for 6 h. Cadmium acetate was used at 10 μM, and all other metal salts were used at 100 μM final concentration. (B) Zinc ion-dependent expression of zitA. An increasing concentration of zinc acetate was added to cultures for induction for 6 h. β-Galactosidase activity was monitored and expressed as nanomoles of o-nitrophenol produced min−1 mg of protein−1. Experiments were performed in triplicate; values are averages, and standard deviations are shown as error bars.
Distribution of CDF family proteins in mycobacteria.
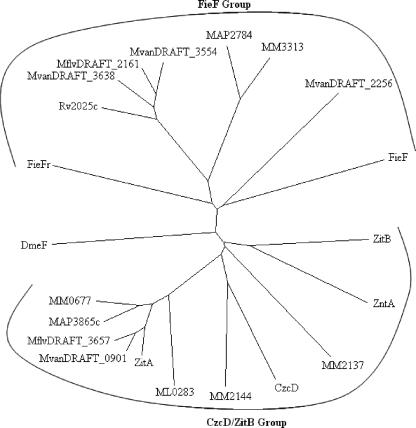
ZitA homologues were searched in completed mycobacterial genomes by BLASTP in the NCBI database and the Mycobacterium marinum genome database at the Sanger Institute (Table 3). Mb2025c from Mycobacterium bovis was identical in sequence with Rv2025c from M. tuberculosis H37Rv, so it was not taken for further analysis. A search for paralogous proteins in the M. smegmatis genome revealed the similarity of MSMEG5964 and MSMEG5963 to the N-terminal and C-terminal regions of CDF proteins, respectively. This indicated that a nonsense mutation in the gene encoding the second CDF of M. smegmatis resulted in two open reading frames with similarity to CDF proteins. Comparative analysis of DNA and translated sequence of the M. smegmatis MSMEG5963/MSMEG5964 region with MAP2784 from Mycobacterium avium subsp. paratuberculosis k10 revealed that a nonsense mutation probably changed codon CAG to stop codon TAG. To confirm that it is not a sequencing error, we amplified this region from M. smegmatis mc2155 and M. smegmatis LR222 and sequenced the amplified product (data not shown). The sequence analysis revealed that both strains had this mutation, indicating that these M. smegmatis strains carry mutations in the second CDF gene. Phylogenetic analysis (Fig. 4) of mycobacterial CDF proteins and characterized bacterial CDF proteins revealed that these proteins cluster in two main groups, the CzcD/ZitB group and the other (FieF) group. M. smegmatis mc2155 possesses only one functional CDF protein, ZitA, which branches with the CzcD/ZitB group along with one homologue each from Mycobacterium leprae TN, M. avium subsp. paratuberculosis k10, Mycobacterium flavescens PYR-GCK, and Mycobacterium vanbaalenii PYR-1, whereas three of the CDF proteins from M. marinum branched with the CzcD/ZitB group. The M. tuberculosis H37Rv CDF protein branched with the FieF group along with one CDF each from M. avium subsp. paratuberculosis k10, M. flavescens PYR-GCK, and M. marinum, whereas three CDF proteins belonged to the FieF group from M. vanbaalenii PYR-1. This analysis revealed the presence of a higher number (two to four) of CDF proteins in environmental and opportunistic pathogenic species, except for M. smegmatis mc2155, which is mutated in the gene coding for the second CDF protein. This mutation might have occurred recently during generation of an electroporation-efficient strain (23), as the gene region still showed a high level of similarity with a homologous region from M. avium subsp. paratuberculosis k10 (data not shown). In comparison, obligate pathogenic species M. tuberculosis H37Rv and M. leprae TN possess only single CDFs. These results indicate that the CDF proteins are required for survival in an external contaminated environment. The presence of more than one member of a particular group may reflect differences in their substrate preference and efficiency (15).
TABLE 3.
Distribution of ZitA homologues in different mycobacterial species
| Organism | No. of homologues | Gene identifier | % Amino acid identity/ similarity |
|---|---|---|---|
| M. marinum | 4 | MM0677 | 66/74 |
| MM2144 | 40/57 | ||
| MM2137 | 30/50 | ||
| MM3313 | 29/45 | ||
| M. vanbaalenii PYR-1 | 4 | MvanDRAFT_0901 | 86/93 |
| MvanDRAFT_2256 | 29/48 | ||
| MvanDRAFT_3638 | 27/39 | ||
| MvanDRAFT_3554 | 24/40 | ||
| M. avium subsp. | 2 | MAP3865c | 77/86 |
| paratuberculosis k10 | MAP2784 | 30/47 | |
| M. flavescens PYR-GCK | 2 | MflvDRAFT_3657 | 85/92 |
| MflvDRAFT_2161 | 26/41 | ||
| M. tuberculosis H37Rv | 1 | Rv2025c | 26/41 |
| M. bovis | 1 | Mb2025c | 26/41 |
| M. leprae TN | 1 | ML0283 | 65/78 |
FIG. 4.
Unrooted phylogenetic tree depicting distribution of ZitA homologues in different Mycobacterium species. Multiple alignments of the sequences were done by ClustalX version 1.81, and the phylogenetic tree was constructed using the neighbor-joining method. The figure was generated from TreeView version 1.6.6. Sequences are as follows (organism name and accession number or gene identifier are in parentheses): ZitA (M. smegmatis, MSMEG0750), MvanDRAFT_0901 (M. vanbaalenii PYR-1, ZP_01208500), MflvDRAFT_3657 (M. flavescens PYR-GCK, ZP_01191608), MAP3865c (M. avium subsp. paratuberculosis k10, NP_962799), MM0677 (M. marinum, MM0677), DmeF (C. metallidurans CH34, RmetDRAFT_5471), FieFr (C. metallidurans CH34, RmetDRAFT_5081), Rv2025c (M. tuberculosis H37Rv, NP_216541), MvanDRAFT_3638 (M. vanbaalenii PYR-1, ZP_01203811), MflvDRAFT_2161 (M. flavescens PYR-GCK, ZP_01193700), MvanDRAFT_3554 (M. vanbaalenii PYR-1, ZP_01203727), MAP2784 (M. avium subsp. paratuberculosis k10, NP_961718), MM3313 (M. marinum, MM3313), MvanDRAFT_2256 (M. vanbaalenii PYR-1, ZP_01207250), FieF (E. coli K12, NP_418350), ZitB (E. coli K12, NP_415273), ZntA (S. aureus, AAC32485), MM2137 (M. marinum, MM2137), CzcD (C. metallidurans CH34, CAA67085), MM2144 (M. marinum, MM2144), and ML0283 (M. leprae TN, NP_301323).
Genes for zitA homologues from other cognitive mycobacterial species, MAP3865c, MAP2784, Rv2025c, and ML0283, were PCR amplified and cloned individually under the control of the hsp60 promoter at PstI and HindIII sites in pSMT3*. Oligonucleotides used for cloning are described in Table 1. MSEMG0750 (zitA) was also cloned in the HindIII site under the control of the hsp60 promoter in pSMT3*, and the construct with correct orientation was checked by restriction digestions. The resultant plasmids pAG3, pAG4, pAG5, pAG6, and pAG7 were electroporated independently in mutant K516 and M. smegmatis mc2155. An M. avium subsp. paratuberculosis k10 zitA orthologue, MAP3865c, partially complemented mutant K516 and increased the zinc acetate MIC to 500 μM in comparison to 150 μM for control (Fig. 5). Another zitA orthologue from M. leprae TN, ML0283, did not result in reversal of the zinc ion sensitivity of the mutant K516 but slightly improved the growth of the mutant at 50 μM zinc acetate. ML0283 is predicted to have a frameshift after codon 220 by GCframeprot and codon usage plot analysis (http://genolist.pasteur.fr/Leproma). The similarity of the encoded protein to known CDFs ends after the probable frameshift, and it is predicted to be nonfunctional. In line with these predictions, our complementation analysis also indicates that the ML0283 gene encodes a defunct protein with very low activity. Homologous CDFs MAP2784 and Rv2025c improved the growth of mutant K516 at 50 μM zinc acetate but failed to provide zinc ion resistance at higher concentrations; nonspecific activity of the translational product of these genes towards different metal ions might be the reason for resistance at lower concentrations of the zinc ions. Many CDF proteins are known to transport more than one metal ion (2, 15, 26). The iron-transporting CDF protein (FieF) and the divalent cation-transporting CDF protein (DmeF) are also shown to transport zinc ions with lower specificity and provide some resistance towards zinc ions (9, 15). Assignment of a precise function to other CDF proteins in mycobacteria needs further functional characterization. The zitA mutant obtained in this study, K516, will be useful for these studies as both the CDF genes are mutated in this strain.
FIG. 5.
Effect of zinc ion on growth of mutant K516 containing zitA mycobacterial homologues. Strains used include M. smegmatis mc2155/pSMT3 (•), K516(zitA::Tn)/pAG7 (as a positive control) (○), K516 (zitA::Tn)/pAG3 (▿), K516(zitA::Tn)/pAG4 (▪), K516(zitA::Tn)/pAG5 (□), K516 (zitA::Tn)/pAG6 (⧫), and K516(zitA::Tn)/pSMT3 (▾). The graph depicts growth in the presence of different concentrations of zinc acetate. The experiment was performed in triplicate; values are averages, and standard deviations are shown as error bars.
Acknowledgments
We thank Samir K. Brahmachari, Director, I.G.I.B., for encouragement and support and S. B. Levy, Eric J. Rubin, K. De Smet, Brigitte Gicquel, Nadine Honore, Makeda Semret, and Y. Singh for M. smegmatis mc2155, pMycoMar, pSMT3*, pJEM13, M. leprae cosmid MLCY789, M. avium subsp. paratuberculosis k10 genomic DNA, and M. tuberculosis H37Rv genomic DNA, respectively. We thank Raj Kishor Kapardar for help in screening and maintenance of the mutant library.
A.G. acknowledges CSIR for a junior research fellowship. This work was supported by grant SR/SO/BB-04/2003 from the Department of Science and Technology (DST), India.
REFERENCES
- 1.Anton, A., A. Weltrowski, C. J. Haney, S. Franke, G. Grass, C. Rensing, and D. H. Nies. 2004. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans CH34 and Escherichia coli. J. Bacteriol. 186:7499-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton, A., C. Grosse, J. Reissmann, T. Pribyl, and D. H. Nies. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canneva, F., M. Branzoni, G. Riccardi, R. Provvedi, and A. Milano. 2005. Rv2358 and FurB: two transcriptional regulators from Mycobacterium tuberculosis which respond to zinc. J. Bacteriol. 187:5837-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, S. M., K. O. Choi, N. Lee, M. Oh, and H. Park. 2006. The zinc chelator, N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine, increases the level of nonfunctional HIF-1α protein in normoxic cells. Biochem. Biophys. Res. Commun. 343:1002-1008. [DOI] [PubMed] [Google Scholar]
- 5.Covarrubias, A. S., A. M. Larsson, M. Högbom, J. Lindberg, T. Bergfors, C. Björkelid, S. L. Mowbray, T. Unge, and T. A. Jones. 2005. Structure and function of carbonic anhydrases from Mycobacterium tuberculosis. J. Biol. Chem. 280:18782-18789. [DOI] [PubMed] [Google Scholar]
- 6.Dean-Ross, D., and C. E. Cerniglia. 1996. Degradation of pyrene by Mycobacterium flavescens. Appl. Microbiol. Biotechnol. 46:307-312. [DOI] [PubMed] [Google Scholar]
- 7.Dussurget, O., G. Stewart, O. Neyrolles, P. Pescher, D. Young, and G. Marchal. 2001. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect. Immun. 69:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grass, G., M. Otto, B. Fricke, C. J. Haney, C. Rensing, D. H. Nies, and D. Munkelt. 2005. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183:9-18. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann, J. L., P. O'Gaora, A. Gallagher, J. E. R. Thole, and D. B. Young. 1996. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 15:3547-3554. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, S. M., G. Grass, C. J. Haney, B. Fan, B. P. Rosen, A. Anton, D. H. Nies, and C. Rensing. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273-278. [DOI] [PubMed] [Google Scholar]
- 12.Leys, N. M., A. Ryngaert, L. Bastiaens, P. Wattiau, E. M. Top, W. Verstraete, and D. Springael. 2005. Occurrence and community composition of fast growing Mycobacterium in soils contaminated with polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 51:375-388. [DOI] [PubMed] [Google Scholar]
- 13.Milano, A., M. Branzoni, F. Canneva, A. Profumo, and G. Riccardi. 2004. The Mycobacterium tuberculosis Rv2358-furB operon is induced by zinc. Res. Microbiol. 155:192-200. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Munkelt, D., G. Grass, and D. H. Nies. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 186:8036-8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton, C. D., M. W. LeChevallier, and J. O. Falkinham III. 2004. Survival of Mycobacterium avium in a model distribution system. Water Res. 38:1457-1466. [DOI] [PubMed] [Google Scholar]
- 17.O'Gaora, P., S. Barnini, C. Hayward, E. Filley, G. Rook, D. Young, and J. Thole. 1997. Mycobacteria as immunogens: development of expression vectors in multiple mycobacterial species. Med. Princ. Pract. 6:91-96. [Google Scholar]
- 18.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199-1210. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen, I. T., and M. H. Saier, Jr. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99-103. [DOI] [PubMed] [Google Scholar]
- 20.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recht, J., A. Martinez, S. Torello, and R. Kolter. 2000. Genetic analysis of sliding motility in Mycobacterium smegmatis. J. Bacteriol. 182:4348-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin, E. J., B. J. Akerley, V. N. Novik, D. J. Lampe, R. N. Husson, and J. J. Mekalanos. 1999. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc. Natl. Acad. Sci. USA 96:1645-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snapper, S. B., R. E. Melton, S. Mustapha, T. Kieser, and W. R. Jacobs, Jr.1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 24.Timm, J., E. M. Lim, and B. Gicquel. 1994. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J. Bacteriol. 176:6749-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkin, J. M., K. Soetaert, M. Stelandre, P. Buyssens, G. Castillo, V. Demoulin, G. Bottu, M. A. Laneelle, M. Daffe, and J. D. Bruyn. 1999. Overexpression, purification and characterization of Mycobacterium bovis BCG alcohol dehydrogenase. Eur. J. Biochem. 262:299-307. [DOI] [PubMed] [Google Scholar]
- 26.Xiong, A., and R. K. Jayaswal. 1998. Molecular characterization of a chromosomal determinant conferring resistance to zinc and cobalt ions in Staphylococcus aureus. J. Bacteriol. 180:4024-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]