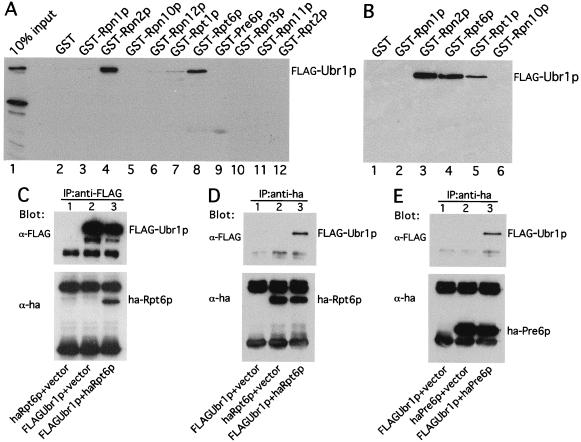
Figure 3.
Ubr1p, the E3 of the N-end rule pathway, is physically associated with the 26S proteasome. (A and B) Ubr1p interacts with Rpn2p, Rpt1p, and Rpt6p in GST-pulldown assays. Extracts of S. cerevisiae containing overexpressed FLAG-Ubr1p (A) or the purified FLAG-Ubr1p protein (B) were incubated with glutathione-agarose beads preloaded with the indicated GST fusions. The retained proteins were eluted, fractionated by SDS/8% PAGE, and immunoblotted with anti-FLAG antibody. Approximately equal amounts of different GST fusions were immobilized on glutathione-agarose beads in these assays, as verified by Coomassie staining (data not shown). (C and D) In vivo association of Ubr1p and Rpt6p. Extracts of S. cerevisiae AVY107 (ubr1Δ) expressing either both FLAG-Ubr1p and HA-Rpt6p, or FLAG-Ubr1p alone, or HA-Rpt6p alone were incubated with anti-FLAG antibody (C) or anti-HA antibody (D). The immunoprecipitated proteins were separated by SDS/12% PAGE and transferred to nitrocellulose membrane. The top halves of C and D show the results of immunoblotting with anti-FLAG antibody; the bottom halves show the analogous data with anti-HA antibody. (E) Coimmunoprecipitation of Pre6p and Ubr1p. Extracts of S. cerevisiae AVY107 (ubr1Δ) expressing both FLAG-Ubr1p and HA-Pre6p, FLAG-Ubr1p alone, or HA-Pre6p alone were incubated with anti-HA antibody, followed by the immunoprecipitation/immunoblotting described in C and D.