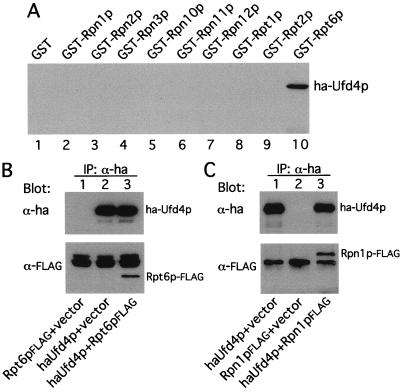
Figure 5.
Ufd4p, the E3 of the UFD pathway, is physically associated with the proteasome. (A) Ufd4p interacts with Rpt6p in the GST-pulldown assay. Extracts of S. cerevisiae containing overexpressed HA-Ufd4p were incubated with glutathione-agarose beads preloaded with different GST fusions, as indicated. The retained proteins were eluted, fractionated by SDS/8% PAGE, and immunoblotted with anti-HA antibody. Approximately equal amounts of different GST fusions were immobilized on glutathione-agarose beads in these assays, as verified by Coomassie staining (data not shown). (B) In vivo association of Ufd4p and Rpt6p. Extracts of S. cerevisiae JD52 (UBR1) expressing both HA-Ufd4p and Rpt6p-FLAG, HA-Ufd4p alone, or Rpt6p-FLAG alone were incubated with anti-HA antibody. The immunoprecipitated proteins were separated by SDS/10% PAGE and transferred to nitrocellulose membrane. (Upper) The results of immunoblotting with anti-HA antibody. (Lower) The data with anti-FLAG antibody. (C) Coimmunoprecipitation of Rpn1p and Ufd4p. Extracts of S. cerevisiae JD52 expressing both HA-Ufd4p and Rpn1p-FLAG, HA-Ufd4p alone, or Rpn1p-FLAG alone were incubated with anti-HA antibody, followed by the immunoprecipitation/immunoblotting procedure described in B, except that SDS/7% PAGE was used.