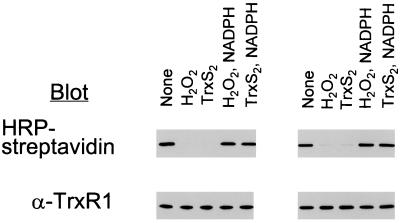
Figure 1.
Effect of exposure of NADPH-reduced TrxR1 to H2O2 or TrxS2 on labeling with BIAM. NADPH-reduced TrxR1 was dialyzed anaerobically to remove NADPH and then incubated for 10 min at room temperature with 200 μM H2O2 or 12 equivalents of TrxS2 per subunit to produce E-H2O2 and E-TrxS2. The H2O2 reaction was stopped by adding catalase, and the E-TrxS2 mixture was adjusted to pH 5.2. One-half of each oxidized enzyme sample was incubated for 20 min with 200 μM NADPH at pH 7.2 to produce E-H2O2-NADPH or E-TrxS2-NADPH. An aliquot (5 μg) of each was incubated with 50 μM BIAM for 10 min in the absence (Left) or presence (Right) of 6 M guanidine-HCl in PBS buffer (pH 7.2) containing 1 mM EDTA and then with 1 mM IAM for 5 min at pH 8.8. The samples shown at Right were dialyzed against 20 mM Tris⋅HCl (pH 8.0) buffer containing 1 mM EDTA to remove guanidine. All samples were subjected to SDS/PAGE on a 10% gel and then transferred to a nitrocellulose membrane. The biotinyl carboxamidomethyl [(B)CAM]-labeled proteins were detected by streptavidin blotting with horseradish peroxidase (HRP)-conjugated streptavidin and enhanced chemiluminescence detection. Equal application of protein among gel lanes was confirmed by immunoblot analysis with antibodies to TrxR1.