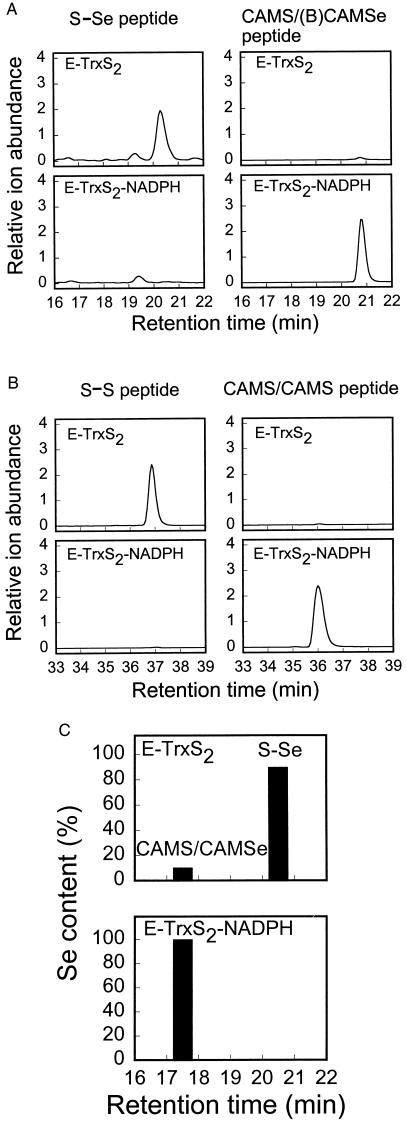
Figure 2.
Identification and quantitation of the Cys497/SeCys498 thioselenide and the Cys59/Cys64 disulfide in E-TrxS2 and E-TrxS2-NADPH. (A and B) E-TrxS2 and E-TrxS2-NADPH were prepared from the NADPH-reduced TrxR1 as described in Fig. 1. The resulting enzymes (10 μg) were labeled with 50 μM BIAM for 20 min in oxygen-free PBS buffer (pH 7.2) containing 1 mM EDTA and then with 1 mM IAM for 5 min in 50 mM Tris⋅HCl (pH 8.8) containing 6 M guanidine-HCl. After adjustment to pH 5.2, the samples were dialyzed for 4 h against 10 mM sodium acetate buffer (pH 5.2) and then for 2 h against 20 mM Tris⋅HCl buffer (pH 8.0). The dialyzed samples were diluted to 10% (vol/vol) in acetonitrile and then incubated with endoproteinase Lys-C at 37°C overnight. The resulting peptide mixtures were analyzed by HPLC-MS. (A) Extracted ion chromatograms for m/z = 649.6 (1,297.7 mu) of the Cys497/SeCys498-containing peptide (Left) and for m/z = 842.5 (1,682.8 mu) of the CAM-Cys497/(B)CAM-SeCys498-containing peptide (Right). (B) Extracted ion chromatograms for m/z = 1,043.9 (3,129.3 mu) of the Cys59/Cys64-containing peptide (Left) and for m/z = 1,082.6 (3,245.5 mu) of the CAM-Cys59/CAM-Cys64-containing peptide (Right). (C) E-TrxS2 and E-TrxS2-NADPH (20 μg) prepared from the NADPH-reduced TrxR1 as described in Fig. 1 were reacted with 1 mM IAM for 5 min in 50 mM Tris⋅HCl (pH 8.8) containing 6 M guanidine-HCl. Lys-C peptides were prepared and fractionated on a C18 HPLC column. Fractions (0.2 ml) were collected manually, and each was analyzed for selenium with a Perkin–Elmer model 4100 ZL atomic absorption spectrometer. The amount of selenium in the total soluble peptide mixture was 100%. The identity of the selenium-containing peptide eluting at 17.5 min was confirmed by matrix-assisted laser desorption ionization time-of-flight MS (measured = 1,413.8 mu; calculated = 1,413.4 mu). When the oxidized peptide eluting at 20.5 min was reduced with DTT, alkylated, and rechromatographed, it eluted at 17.5 min as expected (not shown).