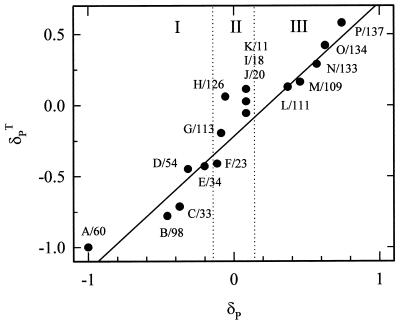
Figure 3.
Correlation between experimental and calculated pseudocontact shifts for the amide groups of the 17 valine residues in the β-chain of the R state structure of Hb. Assignments are based primarily on the alignment of the calculated and experimental chemical shift increments but incorporate results of a [1H,1H]-NOESY-TROSY experiment (Fig. 4) as described in the text. Because only Val-98 is close enough to the α-subunit for its heme to influence δPT, the calculations ignore contributions to the paramagnetism from hemes other than the β-heme. The peaks are classified according to the structural clusters discussed in the text and identified in Fig. 1. The solid line is the best-fit line through the data for the 10 peaks that show a significant experimental shift (slope = 0.92; R2 = 0.97). Cluster II (peaks F–K) falls between the vertical lines that indicate the ±0.05 ppm threshold, below which a paramagnetic-shift increment is not significant, and was excluded from the fit.