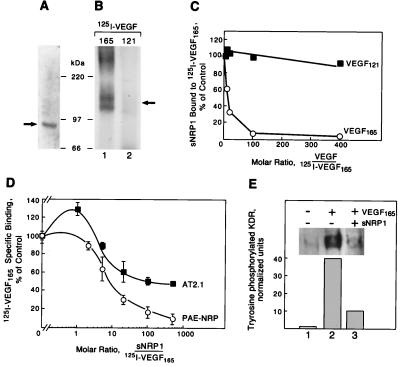
Figure 3.
Recombinant sNRP1 inhibits VEGF165 binding and activity. (A) Recombinant His/Myc-tagged sNRP1 was purified and analyzed by SDS/PAGE and Coomassie blue staining. A single band of approximately 90 kDa is indicated by a solid arrow. (B) sNRP1 (50 ng) was incubated with 5 ng of 125I-VEGF165 (lane 1) or 125I-VEGF121 (lane 2) in solution, followed by cross-linking with disuccinimidyl suberate, SDS/PAGE, and autoradiography. A 120- to 140-kDa complex is formed with 125I-VEGF165 (solid arrow) but not with VEGF121. (C) His/Myc-tagged sNRP1 (500 ng) was incubated in solution with 10 ng of 125I-VEGF165 in the presence of increasing amounts of unlabeled VEGF165 (○) or VEGF121 (■). sNRP1/125I-VEGF165 complexes were immunoprecipitated with anti-Myc antibodies. The relative amounts of sNRP1 bound to 125I-VEGF165 were measured in a γ-counter. The counts were normalized and presented as percentage of control. (D) 125I-VEGF165 (10 ng/ml) was incubated with PAE-NRP1 cells (○) or AT2.1 cells (■) in the absence or the presence of increasing amounts of purified recombinant sNRP1. Cells were lysed and bound 125I-VEGF165 was measured in a γ-counter. Each value represents the average of three separate measurements. (E) PAE/NRP1/KDR cells were starved overnight and either not treated or treated with 5 ng/ml VEGF165 or 5 ng/ml VEGF165 plus 1 μg/ml sNRP1 for 10 min. Cells were lysed and KDR was immunoprecipitated with anti-KDR antibodies. The immunoprecipitated proteins were resolved by SDS/PAGE and analyzed by Western blot with antiphosphotyrosine antibodies. Equal amounts of total protein were present in all samples.