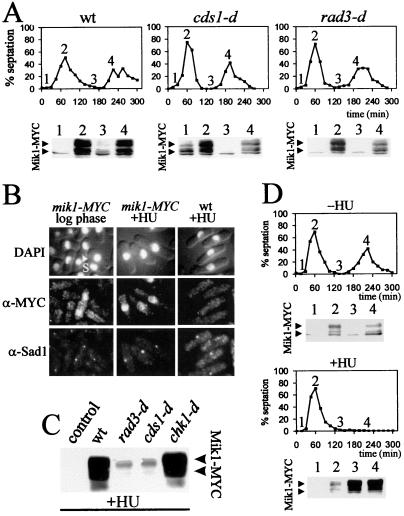
Figure 2.
(A) mik1-MYC cells [in a wild-type (wt), cds1-d, or rad3-d background] were synchronized in G2 by elutriation. Every 15 min, the septation index was monitored. At times indicated by 1 → 4, protein was extracted, and 50 μg (pellet fraction) was analyzed by Western blotting with 9E10. (B) mik1-MYC cells and untagged control cells were grown to mid-log phase (Left) or arrested in S phase by using 20 mM HU for 3.5 h. Cells were fixed and stained for DNA (DAPI), Mik1-MYC (α-Myc), or the spindle pole marker Sad1 (α-Sad1; ref. 15). S indicates a septating S phase cell. Within the unperturbed population, >90% of septated cells were stained. Less than 10% of unseptated cells were stained. (C) Asynchronous mik1-MYC cells (in wild-type, rad3-d, cds1-d, or chk1-d backgrounds) and an untagged control were grown to mid-log phase and arrested in S phase (20 mM HU; 3.5 h), and protein was extracted. Total protein (pellet fraction; 50 μg) was analyzed by Western blotting with 9E10. (D) mik1-MYC cells were synchronized in G2 by elutriation, and the culture was divided in two. HU (20 mM) was added to one half. Samples were taken for septation index every 15 min and for protein extraction and analysis (as above) at the times indicated by 1 → 4.