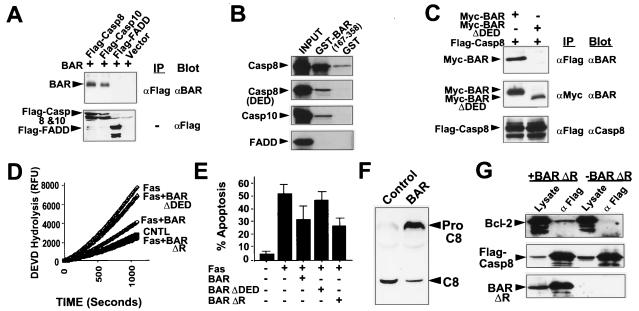
Figure 4.
DED Domain of BAR Is Required for Interactions with Procaspases-8 and -10 and for Inhibition of Fas-induced Apoptosis. (A) Coimmunoprecipitation assays were performed as described above by using 293T cells transfected with plasmids encoding Myc-BAR and either Flag-procaspase-8 (cys/ala), procaspase-10 (cys/ala), or Fadd. (B) For in vitro binding studies, in vitro translated 35S-labeled procaspase-8, procaspase-8 domain (DED), procaspase-10, and FADD proteins were incubated with 10 μg of either GST or GST-BAR-DED (residues 167–358) recombinant proteins. Protein complexes were recovered on glutathione-Sepharose beads, followed by analysis by SDS/PAGE autoradiography. In vitro translation mixes (10% input) were run directly in gels as a control. A wide variety of control GST proteins were tested, thus confirming specificity of BAR interactions with procaspases-8 and -10 (not shown). (C) 293T cells were cotransfected with 5 μg of either BAR- or BAR(ΔDED)-encoding plasmids and 5 μg of a plasmid encoding Flag-procaspase-8. Cell extracts were prepared 2 days later, and immunoprecipitates were prepared by using either anti-Flag or anti-Myc monoclonal antibodies. Immune complexes were subjected to SDS/PAGE immunoblot analysis, by using anti-BAR and anti-procaspase-8 antibodies. (D) For caspase activity assays, 293T cells were transfected with either control plasmid or cotransfected with 0.3 μg of Fas-encoding plasmid and 3.7 μg of either control plasmid or plasmids encoding BAR, BAR(ΔDED) or BAR(ΔR). Cell extracts were prepared 1 day later, normalized for total protein (25 μg), and incubated with 100 μM DEVD-AFC. Enzyme activity was determined by the release of the AFC fluorophore. (E) A portion of the transfected 293T cells described above were stained with DAPI, and the percentage of GFP-positive cells with fragmented or condensed nuclei (apoptotic) was determined (mean ± SD; n = 3). (F) 293T cells were transfected with plasmids encoding procaspase-8 and either pcDNA3 (control) or pcDNA3-Myc-BAR. Cell lysates were prepared 1 day later, normalized for protein content (50 μg), and analyzed by SDS/PAGE immunoblotting by using anti-caspase-8 antiserum. The positions of the unprocessed procaspase-8 and the large subunit of processed caspase-8 are indicated by arrows. (G) 293T cells were cotransfected with 5 μg each of plasmids encoding Bcl-2, Flag-Procaspase-8 and either 10 μg of BAR encoding plasmid (+BAR) or control plasmid (−BAR). Cell extracts were prepared 2 days later and immunoprecipitates were prepared by using anti-Flag antibody. Immune complexes were analyzed by SDS/PAGE immunoblotting, probing the same blot sequentially with anti-Bcl-2 antiserum (Top), anti-Flag monoclonal antibody (Middle), and anti-BAR antiserum (Bottom), with antibody stripping between each detection. Data are representative of several experiments and include a wide variety of control transfections and immunoprecipitations that further confirmed specificity of the observed interactions.