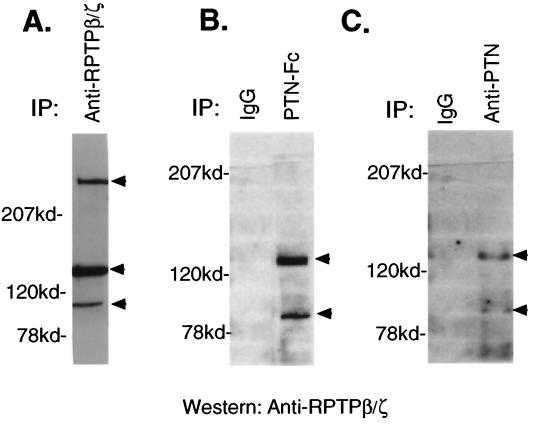
Figure 1.
Association of RPTP β/ζ with PTN. (A) Lysates of U373-MG glioblastoma cells were immunoprecipitated with anti-RPTP β/ζ monoclonal antibodies. The immunoprecipitates were separated on 6% acrylamide gel, transferred to a poly(vinylidene difluoride) membrane, and probed with anti-RPTP β/ζ antibodies. The arrowheads indicate the RPTP β/ζ spliced products of ≈230, 130, and 85 kDa. (B) Western analysis of RPTP β/ζ captured by PTN-Fc. Lysates of U373-MG cells were incubated with PTN-Fc and proteins interactive with PTN-Fc (right lane) were captured with protein A Sepharose-4B beads for 2 h. The beads were washed in cold lysis buffer, boiled in SDS/PAGE sample buffer, and the eluted proteins were separated on an 8% acrylamide gel and analyzed by Western blots probed with anti-RPTP β/ζ monoclonal antibodies. As a control, PTN-Fc was replaced with an equal amount of human IgG (left lane). The arrowheads indicate the ≈130- and ≈85-kDa spliced products of RPTP β/ζ. (C) Western analysis of RPTP β/ζ captured by endogenous PTN. Lysates of U373-MG cells were incubated with anti-PTN monoclonal antibodies (right lane) and the complexes were captured with protein A Sepharose-4B beads for 2 h. The beads were washed in cold lysis buffer and boiled in SDS/PAGE sample buffer, and the eluted proteins were separated on an 8% acrylamide gel and analyzed by Western blots probed with anti-RPTP β/ζ monoclonal antibodies. As a control, mouse IgG replaced the anti-PTN antibody (left lane). The arrowheads indicate the ≈130- and ≈85-kDa spliced products of RPTP β/ζ.