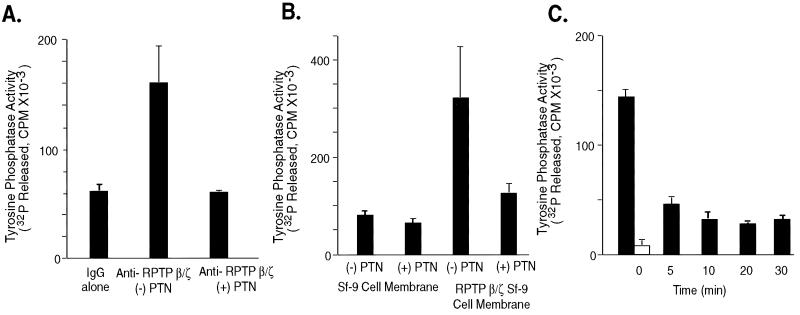
Figure 2.
PTN-dependent inhibition of the intrinsic tyrosine phosphatase activity of RPTP β/ζ. (A) Inhibition of the endogenous RPTP β/ζ tyrosine phosphatase activity in PTN-treated U373-MG cells. The left bar represents tyrosine phosphatase activity in immunoprecipitates from lysates of untreated cells with mouse IgG (control) to replace the anti-RPTP β/ζ antibodies. The center bar represents tyrosine phosphatase activity in immunoprecipitates with anti-RPTP β/ζ antibodies from lysates of untreated cells, and the right bar represents tyrosine phosphatase activity of immunoprecipitates with anti-RPTP β/ζ antibodies from lysates of cells treated with recombinant PTN (50 ng/ml.) (B) Inhibition of recombinant RPTP β/ζ phosphatase activity in Sf9 cell membranes. Membrane fractions of Sf9 cells that were infected by a baculovirus containing a cDNA-encoding RPTP β/ζ (right two bars), or uninfected (left two bars) that were untreated (− PTN) or treated (+ PTN) with 50 ng/ml PTN were assayed as described in Materials and Methods. (C). Time course of PTN-dependent inactivation of RPTP β/ζ in PTN-treated (50 ng/ml) Sf9 cell membranes expressing RPTP β/ζ (solid bars) and Sf9 cell membranes without RPTP β/ζ (open bar, t = 0 only).