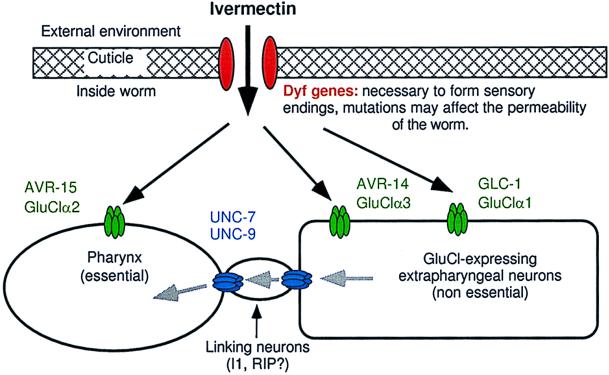
Figure 2.
Preposed model to describe mechanisms of ivermectin sensitivity. Black arrows indicate the diffusion of ivermectin and the gray arrows indicate the flow of ivermectin-induced hyperpolarizing potential. Dyf gene mutations, which may reduce ivermectin permeability, act additively with all combinations of receptor mutants in the tier below. Ivermectin acts independently on each of the GluCl subunits to hyperpolarize cells: GluClα2 (AVR-15) acts in pharyngeal muscle, GluClα3 (AVR-14) acts in neurons. GluClα1 (GLC-1) is presumed to be neuronal but, because we do not know where gcl-1 is expressed, our model is not meant to imply that GluClα1 (GLC-1) and GluClα3 (AVR-14) are necessarily coexpressed or that they do or do not associate to form a channel. The effect of ivermectin on neurons expressing GluCls is not sufficient to kill the worms at low concentrations but requires that the ivermectin-induced hyperpolarization spread via gap junctions encoded by unc-7 and unc-9 to other excitable cells that are essential to the function of the worm. Our results indicate that the spread of hyperpolarization from the extrapharyngeal nervous system back to the pharynx is an important component of the gap-junction-mediated ivermectin sensitivity conferred by GluClα3 (AVR-14) and GluClα1 (GLC-1). The flow of hyperpolarizing potential from the extrapharyngeal neurons to the pharynx occurs via linking neurons such as I1 and RIP that may not themselves express GluCls.