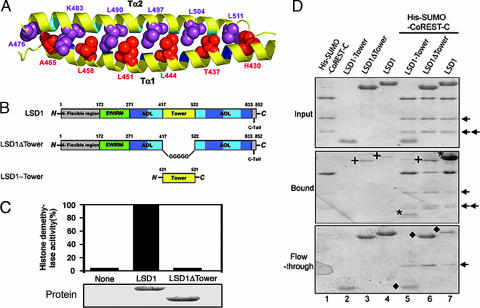
Fig. 5.
The Tower domain of LSD1 is an antiparallel coiled coil and is indispensable for both the demethylase activity and the interaction between LSD1 and CoREST. (A) Ribbon diagram of the coiled coil of the Tower domain. The amino acids at the d positions of the heptad repeat of the two helices are in space-filling representation and colored in red (Tα1) and purple (Tα2), respectively. (B) Schematic diagrams of LSD1 and the LSD1 deletion mutants. The domains are colored as in Fig. 1A. (C) Histone demethylase activity assay of LSD1 and its deletion mutant LSD1ΔTower. Approximately equal amounts (2 μg) of wild-type and mutant recombinant LSD1 (Lower) were incubated with equal amounts of 3H-labeled H3K4 methylated substrates. Released 3H-formaldehyde from each reaction was quantified by scintillation counting (cpm) (Upper). (D) The Tower domain of LSD1 interacts with CoREST. Comparable wild-type and deletion mutants of LSD1 were incubated with Ni2+ beads in the presence or absence of His-SUMO-CoREST-C. The bound proteins (Middle) and the flow-through proteins (Bottom) were analyzed by SDS/PAGE. The input proteins are shown in Top. The asterisks in Middle indicate the bound LSD1 and LSD1-Tower, and the diamonds in Bottom indicate the unbound LSD1 (lane 7) and LSD1 mutants (lanes 5 and 6) after incubation with His-SUMO-CoREST-C. LSD1-Tower did not bind the Ni2+ beads (no LSD1-Tower band in Middle). The crosses indicate the nonspecifically bound LSD1 and LSD1ΔTower (lanes 3, 4, and 6 in Middle). The arrows indicate two degradation products of the His-SUMO-CoREST-C protein.