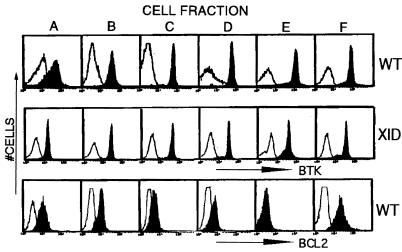
Figure 2.
Btk or Bcl-2 expression during B lineage development. Bone marrow cells from wild-type (Top and Bottom) or xid (Middle) mice were sequentially stained with FITC-conjugated antibodies recognizing B lineage markers (32), and then were fractionated by using either anti-B220 or anti-FITC-conjugated immunomagnetic bead columns. In each step, the marker-positive cells are retained in the magnetic column, and the marker-negative cells are recovered in the flow through fraction. After marrow harvest, erythrocytes were lysed as described in the Methods, and monocytic cells were depleted by using FITC-anti-Mac-1 followed by anti-FITC immunomagnetic bead columns. The first purification step was performed by staining Mac-1− marrow cells with FITC-anti-IgD to obtain fraction “F” (IgD+ cells). The IgD− cell population was then stained with FITC-anti-IgM to yield fraction “E” (IgM+). The IgD−IgM− cell population was immunostained with FITC-anti-BP-1. BP-1+ cells were next stained with FITC-anti-CD43 whereas BP-1− cells were stained with FITC-anti-HSA. A positive selection with anti-B220 immunomagnetic beads was used to recover B lineage cells from these latter steps to yield the final four cell fractions: BP-1−, HSA−, B220+ (“A”); BP-1−, HSA+, B220+ (“B”); BP-1+, CD43+, B220+ (“C”); BP-1+, CD43−, B220+ (“D”). Purified cells were fixed and immunostained with a nonspecific antibody (open histograms), anti-Btk [filled histograms (Top and Middle)], or anti-Bcl-2 [filled histograms (Lower)] followed by the appropriate fluorescent-tagged secondary antibody.