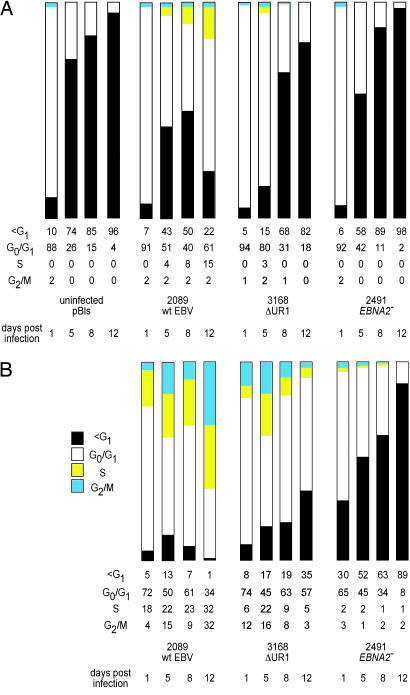
Fig. 2.
Primary B cells infected with the ΔUR1-EBNA1 mutant EBV cease to proliferate 1 week p.i. (A) The cell cycle status of the entire population of infected and uninfected primary B cells was determined with the thymidine analogue BrdU, which was added for 2 h before FACS analysis. The cells were stained with an APC-coupled BrdU-specific antibody, and the cellular DNA was counterstained with the DNA intercalating dye 7-AAD. Cells were analyzed for G1, S, and G2/M phases of the cell cycle and for subG1 DNA content. FACS analyses were performed until a total of 3 × 104 cells were collected and set to 100%. Uninfected cells and cells infected with the EBNA2− mutant 2491 EBV did not enter the cell cycle and became apoptotic by day 5 p.i., as indicated by their subG1 DNA content. Cells infected with the ΔUR1-EBNA1 mutant EBV initially entered the cell cycle, as indicated by a small fraction of cells in S phase, but later ceased to proliferate. (B) To exclude cellular debris, the EBV-infected cells were gated for forward and sideward scatter, and only GFP+ cells within the lymphocyte gate were considered for analysis. Wild-type 2089 EBV induced progression in the cell cycle as early as 24 h p.i., and the fraction of cells in S or G2/M phase increased from that time point onward. Primary B cells infected with 3168 (ΔUR1-EBNA1) were indistinguishable from wild-type EBV-infected cells until day 5 p.i. At days 8 and 12 p.i., the majority of ΔUR1-EBNA1 EBV-infected cells ceased to proliferate, and the fraction of cells with a subG1 DNA content increased considerably. Cells infected with the EBNA2− mutant 2491 EBV did not enter the cell cycle and became increasingly apoptotic, as indicated by their subG1 DNA content. One representative experiment of three is shown.