Abstract
By using rational design, antibody fragments (Fabs) that mimic thrombopoietin (TPO) were created. A peptide with cMpl receptor-binding capability was grafted into different complementarity-determining regions of a fully human Fab scaffold. Functional presentation of the peptide was optimized by using phage display and cell-based panning. Select antibodies and fragments containing two grafted peptides were assayed for their ability to stimulate the cMpl receptor in vitro. Several candidates demonstrated agonist activity in an in vitro cMpl receptor signaling reporter assay, including Fab59, which was estimated to be equipotent to TPO. Fab59 additionally was able to effectively stimulate platelet production in normal mice. These rationally designed mimetic Fabs may provide a therapeutic intervention for thrombocytopenia while avoiding the potential generation of neutralizing antibodies to endogenous TPO. Furthermore, this study demonstrates a method by which short-lived linear peptides with binding activity may be converted to more stable and potent agonists capable of activating cell surface receptors.
Keywords: cMpl receptor
Thrombocytopenia, or low platelet count, is a significant clinical problem associated with the chemotherapeutic treatment of cancer patients. Although transfusion therapy is generally effective in dealing with thrombocytopenia, significant cost and risks are associated, including the transmission of infectious agents, the development of platelet-reactive alloantibodies, and transfusion reactions (1). Concerns over these complications have led to the development of agents that stimulate platelet production and reduce the need for transfusions. Such agents may benefit patients with chemotherapy-induced thrombocytopenia and those with other forms of thrombocytopenia, including idiopathic thrombocytopenic purpura, myelodysplastic syndrome, chronic liver disease, or AIDS (2). Molecules that increase platelet counts include recombinant IL-11 [Neumega (Wyeth, Madison, NJ); refs. 2 and 3], recombinant forms of thrombopoietin (TPO; refs. 2 and 3), and a variety of TPO mimetics (4–10).
Although IL-11 has been shown to reduce platelet transfusions, its pleiotropic effects contribute to undesirable side effects limiting its use as a thrombopoietic agent (2, 11). Unlike IL-11, the cytokine TPO acts in a lineage-specific manner and is an important physiologic regulator of platelet production. TPO binds to the cMpl receptor (cMpl-R) on megakaryocytic progenitors and stimulates proliferation and differentiation leading to increased platelet production. Clinical trials with recombinant versions of TPO have been shown to increase platelet counts in humans with normal bone marrow and to benefit patients receiving nonmyeloablative chemotherapy (12–14). However, two different recombinant forms of TPO used in clinical trials have caused an immune response and the development of antibodies that recognize endogenous TPO (2, 15, 16). The possible generation of anti-TPO antibodies that impair the function of the endogenous cytokine is a significant disadvantage of this treatment approach.
Alternate thrombopoietic agents that lack native TPO primary sequences have been developed to address this concern. Peptide mimetics that bind and activate the cMpl-R have been identified, including one with potency equal to that of recombinant human TPO (rhTPO) in cell-based assays (4). Immunogenicity studies in animals suggested that immune responses directed against such mimetics would not crossreact with native TPO in humans (17), establishing that a TPO mimetic could be a safe therapeutic strategy. Alternate TPO mimetic peptides (6), mimetic peptide fusions on Fc (7, 8), agonist minibodies (9), and small molecule mimetics (10) have also been reported.
After the report of strong agonistic activity by a chemically dimerized peptide mimetic (4), we undertook an approach designed to extend the half-life and thereby convert the peptides into a potential therapeutic agent. We have altered complementarity-determining regions (CDRs) of a fully human antibody to present two copies of the peptide. The amino acids flanking the peptide required optimization for proper presentation of the peptide in the context of the antibody scaffold. Clones created represent a unique class of antibody agonists capable of binding and activating the cMpl-R to stimulate growth in cell-based assays and platelet production in mice. This technology represents an innovative approach by which short-lived linear peptides with binding capabilities may be converted to rationally designed agonist antibodies with therapeutic potential.
Results
cMpl-R Binding by an Antibody Scaffold Containing a Single Peptide.
The linear 14-aa peptide AF12505 (IEGPTLRQWLAARA) binds to the cMpl-R as a monomer, and when chemically dimerized, the activity of the dimer (AF13948) is equipotent to the native cytokine TPO (EC50 = 100 pM; ref. 4). To convert the peptide into a potential therapeutic, we used a peptide grafting approach within a human antibody scaffold. Receptor binding was evaluated after grafting of the AF12505 peptide sequence into the heavy chain (HC) CDR3 (18) of a well characterized human anti-tetanus toxoid (TT) Fab (19–21). This antibody was selected as a scaffold, because its TT-binding specificity could be switched by simply replacing the HC-CDR3 (20, 21). To achieve optimal presentation of the peptide within the Fab scaffold, two amino acids flanking each end of the peptide were randomized, resulting in the generation of an antibody library in which Fab constructs were identical except for the four randomized amino acids flanking the grafted peptide. The Fab library was selected for binding to the cMpl-R in its native conformation on cell surfaces, which was accomplished by using phage-displayed Fab libraries and cell-based panning (22). The Fab library containing random residues flanking the grafted peptide in the HC-CDR3 was panned on human platelets.
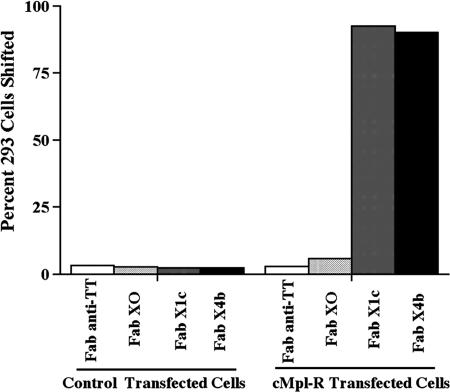
After panning, Fabs were tested as pools of three clones by FACS analysis on human platelets. Pools demonstrating binding were then deconvoluted by testing the individual Fab clones for binding to the megakaryocytic cell line CMK (German Collection of Microorganisms and Cell Cultures, www.dsmz.de) that naturally expresses cMpl-R (data not shown). Fig. 1 demonstrates that binding was occurring specifically to the cMpl-R by comparing FACS analysis performed on 293 Epstein–Barr virus-encoded nuclear antigen (EBNA) cells transfected with either the cMpl-R or an empty control vector. Additionally, the peptide-grafted Fabs did not bind to the parental Fab's antigen, TT (data not shown). Individual clones, such as clones FabX1c, FabX4b, and X4c, with strong specific binding to cMpl-R were sequenced, revealing a strong selection for a proline at the flanking position immediately downstream of the peptide. For comparison, a number of clones with weak binding were randomly selected and sequenced. Despite the presence of the cMpl-R-binding peptide in these clones, binding by FACS was extremely weak. None of these randomly picked clones contained the characteristic proline in the immediate downstream flanking position. (For a table listing the sequences of representative strong and weak binding clones, see Table 2, which is published as supporting information on the PNAS web site.) Furthermore, an individual clone concurrently constructed to contain glycines in the four flanking positions also showed extremely weak binding by FACS (FabX0). These data suggest that mere placement of the peptide within this scaffold was insufficient for optimal presentation and strong binding to the cMpl-R. In contrast, clones with a proline in the immediate downstream position presented the peptide in a preferred conformation for binding and had a strong selective advantage during the four rounds of panning.
Fig. 1.
Analysis of cMpl-R-binding Fabs expressed as soluble proteins. FACS analysis of Fabs expressed in bacterial supernatant on 293 EBNA cells +/− cMpl-R transfection. The parental scaffold Fab (anti-TT) used for peptide grafting was tested as a negative control. Representative Fabs containing a single cMpl-R-binding peptide in HC CDR3 (Fabs X0, X1c, and X4b) differ only by flanking amino acids.
Agonist Activity by Crosslinked Antibody Fragments.
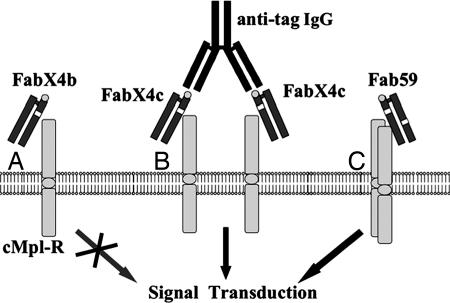
The c-Mpl-R belongs to the hematopoietic superfamily of receptors in which activation requires a signaling-competent dimer. Indeed, data support that efficient cMpl-R activation requires a ligand with two receptor-binding domains (4, 23). Thus, selected peptide-grafted Fabs were converted into dimeric binding structures and evaluated for agonist activity. Dimeric binding of the Fabs was initially achieved by crosslinking via a secondary antibody, 12CA5, that recognizes the HA tag (24) at the C terminus of the HC (Fig. 2B). Fabs crosslinked by 12CA5 were evaluated in a transcription-based assay to monitor cMpl-R activation. In this assay, luciferase expression is driven by the c-Fos promoter. This reporter system is biologically relevant, because native TPO stimulation of cMpl-R leads to activation of the MAPK pathway causing transcription of c-Fos (25). This assay requires only a short induction period (6 h), making it amenable for testing bacterial supernatants containing unpurified Fab. Receptor activation was observed upon addition of increasing amounts of 12CA5 to FabX4c bacterial supernatant (see Fig. 5, which is published as supporting information on the PNAS web site), whereas addition of 12CA5 alone to the cMpl-R-transfected 3T3 cells did not stimulate activity (data not shown). Because no activity was observed in the control vector-transfected cells treated equivalently, the observed stimulation was specific for cells expressing the cMpl-R. These results indicated that dimerization of selected Fabs was sufficient to stimulate the cMpl-R in these assays.
Fig. 2.
Schematic representation of the proposed interaction of Fab fragments with cMpl-R. The white box within the Fab represents an inserted cMpl-R-binding peptide. The Fab HA epitope tag on the HC is represented as a gray circle. (A) Fab with a single cMpl-R-binding peptide interacting with the receptor. (B) Crosslinking of single peptide containing Fabs having an HA tag with anti-HA secondary antibody. (C) Fab with two cMpl-R-binding peptides interacting with two receptors.
To further characterize receptor activation in the context of a divalent antibody, more stable divalent antibody structures were created and evaluated. Whole IgG and F(ab′)2 formats that contained a cMpl-R-binding peptide in each HC-CDR3 were analyzed for agonistic activity. Both were found to have ≈2 orders of magnitude lower activity relative to rhTPO (Table 1) or the chemically linked dipeptide AF13948. Although activity was induced by the grafted IgG or F(ab′)2, we hypothesized that if activation was occurring by receptor homodimerization, the orientation and/or distance between two receptors held in a complex may contribute to suboptimal receptor activation.
Table 1.
Comparison of antibody format, peptide insertion, and relative agonistic activities based on the in vitro cMpl-R luciferase reporter assay
| Antibody construct | cMpl-R-binding peptide position | Activity, IC50 |
|---|---|---|
| rhTPO | Not applicable | 100 pM |
| Full IgG | Two HC CDR3s | 20 nM |
| F(ab')2 | Two HC CDR3s | 20 nM |
| Fab 59 | HC CDR3 and LC CDR2 | 100 pM |
| Fab 16C/X4 | HC CDR3 and LC CDR1 | >30 nM |
Agonist Activity by Fabs Containing Multiple Peptides.
To address our hypothesis on the relationship between the distance separating two receptors in a complex and receptor activation, we chose to test whether more optimal receptor activation could be achieved by using a “divalent Fab”, or more specifically, a single Fab with two peptides contained in two different CDRs. This strategy would bring the peptides grafted into a Fab closer together, potentially allowing more native cMpl-R association as a homodimer.
Optimal presentation of the peptide was determined for each CDR position (18) in independent panning experiments. To achieve this result, additional Fab libraries were constructed in which the cMpl-R-binding peptide was inserted into different CDRs of the anti-TT Fab. Again, each CDR was replaced by the peptide flanked by two randomized amino acid positions on each end. Libraries were panned separately on cMpl-R-transfected cells. Individual Fabs were tested for binding by FACS analysis on cMpl-R-transfected 293 EBNA cells. Clones with strong binding specific for the cMpl-R were obtained from the HC CDR2, light-chain (LC) CDR1, and LC CDR2 libraries. DNA sequence analysis revealed that selection for preferred flanking amino acids had occurred. Fab clones from the LC CDR1 library had a consensus of R-G-peptide-V-A, whereas clones from the LC CDR2 library had a consensus of N-P-peptide-R-G. No consensus within the randomized flanking amino acids was observed for clones from the HC CDR2 library (see Table 2).
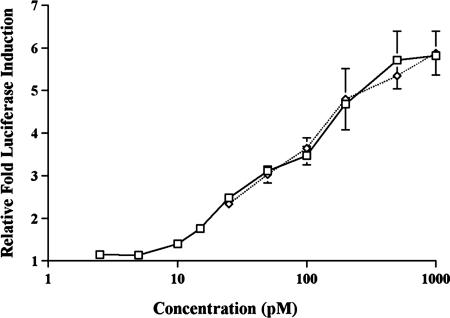
Grafting combinations of the selected peptides plus their respective flanking residues into two different CDR regions within a single Fab domain created divalent Fabs (Fig. 2C), which were examined in the luciferase reporter assay. Strong agonist activity was obtained from divalent Fabs with peptides in the HC CDR2 and HC CDR3 positions and from divalent Fabs with peptides in the HC CDR3 and LC CDR2 positions. The specific activity of one such clone, Fab59, was examined in more detail after a two-step purification (anti-Fab affinity column and size exclusion column) and estimated to be equipotent to rhTPO (Fig. 3) and similar to that reported for the dimeric peptide mimetic AF13948 (4). In contrast, clones with peptides in the LC CDR1 and HC CDR3 position had very low TPO mimetic activity, estimated to be >300-fold below that of the native cytokine (Table 1).
Fig. 3.
Titration of agonist activity of purified Fab 59 using the luciferase reporter assay (described in Materials and Methods). Fab 59 contained two cMpl-R-binding peptides, HC CDR3 clone X4c and LC CDR2 clone LR2–19. NIH 3T3 cells were transfected with cMpl-R and the Fos promoter/luciferase reporter construct. Fab59 (diamond) and rhTPO (square) were titrated for activity testing.
Additional Characterization of Fab59.
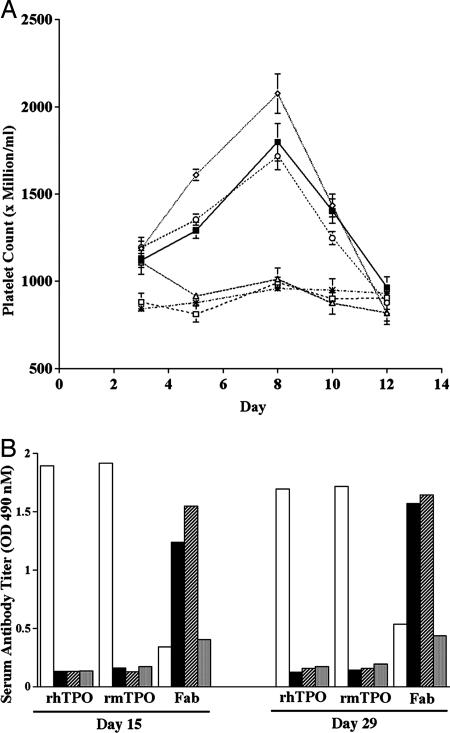
To test Fab59 in vivo, normal mice were injected daily with either purified rhTPO (90 μg/kg), purified monomers of Fab59 (0.2, 2, or 5 mg/kg), or negative control anti-TT Fab (5 mg/kg) over a 5-day time course. The selected doses of Fab59 represent 3×, 30×, and 75× molar doses when compared with rhTPO. Treatment with either Fab59 or rhTPO induced a comparable rise in peripheral platelet counts (Fig. 4A) relative to that observed in the control mice, although higher doses of Fab 59 were required. The ≈30-fold reduced potency in vivo compared with our in vitro studies may be the result of differences between mouse and human receptor or may simply reflect the half-life and clearance of the Fab compared with rhTPO. Serum antibodies generated in response to dosing with Fab59, rhTPO, and anti-TT Fab were evaluated at days 15 and 29 for reactivity to human Fab, rhTPO, and recombinant murine TPO (rmTPO; Fig. 4B). As expected, an immune response against the human antibodies Fab59 and anti-TT Fab was generated after repeat administration in mice. However, significantly, we observed that the antibodies did not crossreact with murine or human TPO.
Fig. 4.
In vivo activity of Fab59. (A) Platelet stimulation in normal mice treated s.c. twice per day with Fab59: 5 mg/kg (open diamond), 2 mg/kg (open circle), 0.2 mg/kg (open triangle), 90 μg/kg rhTPO (filled square), or 5 mg/kg negative control anti-TT Fab (open square) for 5 consecutive days. Platelet levels of untreated mice are also shown (asterisks). (B) Serum antibody responses on days 15 and 29 after dosing mice as described above for 5 consecutive days with rhTPO (white bar), 5 mg/kg for Fab59 (black bar) or negative control anti-TT Fab (striped bar). Serum reactivity for untreated mice is also shown (gray bar). All sera were screened for binding to immobilized rhTPO, rmTPO, and human Fab (x axis) in a direct ELISA.
Discussion
Using a rational design strategy, agonist antibody fragments were created that are capable of mimicking TPO. A small short-lived peptide, capable of binding the cMpl-R, was grafted into two different CDRs of a fully human Fab. Similar to rhTPO, the resultant Fab products were capable of activating the cMpl-R both in vitro and in vivo. These results demonstrate that the dipeptide, if presented properly, is able to maintain its activity even in the context of a different environment.
Amino acids flanking the peptide strongly influenced the ability of the peptide to bind to the cMpl-R. Selection for consensus sequences in the randomized flanking positions was observed when the peptide was placed in the HC CDR3, LC CDR2, and LC CDR1. Although no consensus was selected when the peptide was placed in the HC CDR2 position, flanking amino acids had an effect on binding as observed by FACS (data not shown). Despite numerous attempts, no cMpl-R-binding clones were obtained when the peptide was placed in the LC CDR3, suggesting that the binding sequences were not accessible in this CDR position.
The luciferase reporter assay served as a first screen to evaluate candidates. Combinations of two cMpl-R-binding peptides within an antibody framework resulted in greatly varied agonist activity in this assay (Table 1). Increased activity was obtained when the peptides were placed in close proximity by using different CDRs within the same Fab. This result indicates that in some cases the binding, dimerization, and activation of the receptors may not be sufficiently achieved when the receptors are bound by the two separate arms of an antibody, as found in F(ab′)2 and IgG constructs.
We believe our rational design approach for the generation of agonist antibodies has several advantages over other TPO mimetics. Using our approach, we can extend the half-life of an agonist peptide by grafting it into a human antibody framework in regions that are known for diversity. Because peptides generally have very short serum half-lives, additional modifications are often required to be therapeutically effective in vivo. Such modifications may alter the peptide activity or safety profile. An additional advantage of our approach is that, in contrast to peptide-Fc fusion agonists in which the IgG constant region may retain effector function, our Fab lacks C region-mediated effector function. Because the peptide targets the receptor on the surface of megakaryocyte precursors, megakaryocytes, and platelets, C region-mediated effector function could have a detrimental effect (ADCC, CDC, etc.) on the same population of cells targeted for stimulation. In comparison to small-molecule mimetics, which could have the therapeutic advantage of reduced cost of goods and ease of administration, the rate-limiting step in their clinical and commercial development is often in associated nonspecific toxicities. In our approach, we are extending the half-life of the peptide by grafting it into a human antibody framework in regions that are known for diversity; the Fab lacks effector function, and antibodies and antibody fragments in general have demonstrated good safety profiles in a variety of disease indications in the clinic.
This work demonstrates that agonist antibodies can be created with strong biological activity by using rational design methods. Human antibodies represent an excellent scaffold in which to graft peptides for the creation of human antibody therapeutics. Not only do they confer greater stability and longer half-life to a peptide, but the six CDRs per Fab arm provide a flexible platform in which to graft various dipeptide configurations for the evaluation of agonist activity. Human Fabs have been shown to change specificity upon conversion of the HC CDR3, yet because of the natural variability within this region, the rest of the antibody structure is likely to remain the same. Peptides have previously been grafted into antibodies and acted as antagonists (26). Using our approach, linear peptides with no biological activity other than their binding capabilities can be potentially converted to agonists with strong biological activity. A number of known peptides with protein-binding capabilities have been identified (27, 28). Using these and other peptides, or peptide mimetics, this method can potentially be applied to a number of disease targets to create agonist antibodies for human therapeutics.
The agonist antibodies described herein may provide therapeutic benefit in humans suffering from thrombocytopenia. The results of previous clinical trials evaluating the efficacy of recombinant versions of human TPO for the treatment of thrombocytopenia have been disappointing, with reports of immunogenicity and the development of anti-TPO antibodies in treated patients. Immunogenicity problems identified in previous human studies by using pegylated megakaryocyte growth and development factor (PEG-MGDF) arose when the immune response generated against the drug also recognized the endogenous human cytokine and led to severe bleeding episodes. Our approach has addressed this problem by creating an agonist lacking sequence homology to TPO, which should eliminate the potential to generate an immune response that can crossreact with endogenous TPO. The immune response in mice against human Fab59 after repeated dosage was tested. Mice serve as a relevant animal model to evaluate immunogenicity, because native mouse and human TPO is known to have good crossspecies activity; we have demonstrated that our candidate mimetic also shows good cross-species activity. Importantly, these characteristics allow us to evaluate whether an immune response against our candidate mimetic will affect the activity of the endogenous murine cytokine in the in vivo model. As expected, we have demonstrated that in mice, an immune response against the human Fab is generated after repeat administration. However, we observed that the antibodies do not crossreact with murine or human TPO. Although we cannot rule out the possibility of immunogenicity in humans before the initiation of human studies, sequences in the CDRs, particularly the HC CDR3, are highly variable in nature by design; therefore, we do not anticipate immunogenic problems with insertion of our peptides into the CDRs of an antibody. Importantly, these data do suggest, however, that if an immune response were generated in humans after repeat administration of our mimetic, it would be unlikely to affect the activity of the endogenous human cytokine.
Materials and Methods
Reagents and Cell Culture.
DMEM, RPMI medium 1640, FBS, and other cell culture reagents were from Invitrogen/Gibco (San Marcos, CA). Effectine transfection reagent was from Qiagen (Valencia, CA). 12CA5 anti-HA was from Roche Molecular Biochemicals (Indianapolis, IN). Rat high-affinity anti-HA (clone 3F10) was from Roche Molecular Biochemicals. Monoclonal mouse anti-human MPL and rmTPO were from R&D Systems (Minneapolis, MN). rhTPO for in vitro assays was from R&D Systems and for in vivo modeling was generated in house (see below). Platelets were from the San Diego Blood Bank (San Diego, CA). CMK cells were cultured in RPMI medium 1640 supplemented with 20% FBS. NIH 3T3 and 293 EBNA cells were cultured in DMEM supplemented with 10% FBS. The nickel-chelate chromatography resin was from Qiagen.
Library Construction.
Phage display vector pRL4, which is a modified version of pComb3H (22), was used for library construction and panning. The anti-TT variable heavy chain sequence was modified at the N terminus of the HC so that human consensus sequence EVQL rather than the previous murine sequence of QVKL was present. The cMpl-R-binding peptide IEGPTLRQWLAARA, flanked by two random amino acid positions each side, was grafted into the anti-TT Fab HCDR3. The DNA sequences coding for the Fab were generated by using overlap extension PCR with TaqDNA polymerase (PerkinElmer, Wellesley, MA). The Fab products were SfiI-digested, ligated into pRL4, and electroporated into competent ER2537 bacteria (suppressor strain; New England Biolabs, Ipswich, MA). Methods used for growth of the bacteria, infection with helper phage, and phage precipitation were as described (22), except that the phage pellet was resuspended in 1%BSA/PBS, filtered, and dialyzed against PBS. The construction of Fabs containing the nonrandomly linked peptide was performed as described above, substituting with alternate specific primers. Four additional libraries were constructed separately, replacing the HC CDR2 or the LC CDR1, CDR2, and CDR3 with the cMpl-R-binding peptide flanked by two random amino acids by using the NNK doping strategy. The generation of the libraries was similar to that described for the HC CDR3 library above (additional details are provided in Supporting Text, which is published as supporting information on the PNAS web site).
Pooled Cloning of Fabs with Two cMpl-R-Binding Peptides.
Combinations where one of the cMpl-R-binding peptides was in the LC and the other was in the HC were made by using the pooled plasmid DNAs and the unique restriction sites flanking the HC (XhoI-SpeI) and LC (SacI-XbaI). Combinations where two cMpl-R-binding peptides were combined within a given chain were performed by using overlap PCR to generate the fragments for cloning. PCR products were purified, then combined in an overlap PCR, where fusion of the two fragments occurred through the complementary FR3 sequences. The full HC product was cloned by XhoI/SpeI into a plasmid containing the anti-TT LC.
Panning on Human Platelets and cMpl-R-Transfected Cells.
Initial cell-based panning was performed on human platelets. To 1 ml of concentrated human platelets from the San Diego Blood Bank, 50 μl of freshly prepared Fab-phage was added in a 15-ml conical tube with 0.1% NaN3. The tube was mixed at room temperature (RT) for 1–2 h. Ten milliliters of 50% human serum + 50% [Iscove's Modified Dulbecco's Medium (IMDM)/10% FBS/0.1% azide/2 mM EDTA] was added to the phage/cells. Platelets were pelleted at 5,500 × g for 5 min at RT. Supernatant was drained, and the pellet was left resting under ≈500 μl of the wash for 20 min. The platelets were very gently resuspended, and then 10 ml of 25% human serum + 75% (IMDM/10% FBS/0.1% azide/2 mM EDTA) was added to the phage/cells. Centrifugation, pellet rest, and resuspension of the platelets were repeated. In panning rounds three and four, a third wash was performed. The washed phage/cells were transferred to a 1.5-ml Eppendorf tube and spun at 5,200 × g. Phage were eluted from the platelets by using glycine buffer (pH 2.2) and then amplified overnight as described in Library Construction. Because of the variable levels of cMpl-R on platelets received from the San Diego Blood Bank, as well as safety risks associated with handling human blood products, subsequent panning experiments were done on cMpl-R-transfected cell. For a detailed protocol, see Supporting Text.
Luciferase Assays.
For luciferase assay-based measurements of agonist activity, NIH 3T3 cells were cotransfected with either a control vector (pcDNA3.1, Invitrogen, Carlsbad, CA) or the cMpl-R expressed in pcDNA3.1 and the Fos promoter/luciferase reporter construct [−710 to +44 of the c-fos promoter (29) cloned into a firefly luciferase reporter (Promega, Madison, WI)]. Cotransfections of 3T3 cells were performed by plating NIH 3T3 cells at 3 × 105 cells per 6-cm dish and then transfecting the next day. NIH 3T3 cells were transfected by using the Effectine lipofection reagent (Qiagen), transfecting each plate with 0.1 μg of pEGFP (Clontech)/0.2 μg of the Fos promoter/luciferase construct/0.2 μg of pAdVAntage/0.5 μg of either the empty control vector pcDNA3.1 (Invitrogen) or the plasmid expressing the cMpl-R. 3T3 cells were placed in 0.5% serum 24 h posttransfection and incubated for an additional 24 h in this low-serum medium to serum starve the cells to reduce the background activation of the Fos promoter. Antibody, either as bacterial supernatants or purified, was then applied to these cells for 6 h. All raw bacterial supernatants were pH-adjusted with 1 M NaOH (21 μl/ml) before addition to the tissue culture media. Cells were harvested and luciferase assays performed by using 50 μg of cell lysate with a Turner Designs TD20/20 luminometer and beetle luciferin (Promega).
FACS Analysis of cMpl-R-Binding Fabs.
Fabs were expressed as soluble proteins in nonsuppressor bacterial strain TOP10F′ (Invitrogen). Pools of three Fabs were tested for binding to human platelets after platelet panning. In initial screens 25 μl of concentrated human platelets (San Diego Blood Bank) were washed once with 1 ml of PBS/5 mM EDTA/2% FBS and then incubated with 100 μl of bacterial supernatant containing the primary (1°) Fab antibodies (20 μl of bacterial supernatant from each of three Fabs preblocked with 40 μl of 5% milk/PBS) at RT for 20 min, followed by a 30-min incubation with secondary (2°) anti-HA antibody 3F10 (Roche Molecular Biochemicals) and a 20-min incubation in the dark with tertiary (3°) anti-rat IgG-FITC antibody (Sigma, St. Louis, MO) in PBS/1% BSA/0.1% NaN3. Cells were washed with 1 ml of FACS buffer and centrifuged between each incubation step. Cells were resuspended in 200 μl of FACS buffer for FACS analysis
Fabs were also tested for binding to the cMpl-R expressed on the megakaryocytic cell line CMK or cMpl-R-transfected 293-EBNA cells. Cells (1 × 106) were incubated with the 1° Fab antibody (bacterial supernatant from each Fab clone preblocked with 5% milk/PBS) and further stained for FACS analysis as described above, except that a 3° anti-rat lgG–phycoerythrin antibody (Research Diagnostics, Concord, MA) was used.
Fermentation.
Bacterial fermentation was performed under fed-batch conditions in a 5-liter Fermentor (Bio-Stat B, Bio-Stat, Stockport, U.K.). One-liter complex fermentation medium contained 5 g of yeast extract, 20 g of tryptone, 0.5 g of NaCl, 4.25 g of KH2PO4, 4.25 g of K2HPO4·3H2O, 8 g of glucose, 2 g of MgSO4·7H2O, and 3 ml of trace metal solution (2.7% FeCl3·6H2O/0.2% ZnCl2·4H2O/0.2% CoCl2·6H2O/0.15% Na2MoO4·2H2O/0.1% CaCl2·2H2O/0.1% CuCl2/0.05% H3BO3/3.7% HCl). The fermentor was inoculated with an overnight culture (5% vol/vol) and grown at constant operating conditions of pH 6.9 (controlled with ammonium hydroxide and phosphoric acid) and at 30°C. The airflow rate and agitation were varied to maintain a minimum dissolved oxygen level of 40%. The feed (with 40% glucose) was initiated once the glucose level in the culture was below 1 g per liter, and the glucose level was maintained at 0.5 g per liter for the rest of the fermentation run. When the OD600 reached ≈18, isopropyl β-d-thiogalactoside was added into the culture to a final concentration of 0.05 mM. Four hours after induction, the fermentor was harvested. The bacterial pellet was centrifuged down and stored at −80°C for future purification.
Fab59 Purification.
Bacterial pellets were suspended into 1× pellet volume PBS buffer, pH 7.0. After gentle sonication, samples were centrifuged at 31,000 × g at 4°C for 15 min. The supernatant was collected and filtered through 0.8- and then 0.2-μm filtration units (NALGENE, Apogene Technologies, McLean, VA). Fab59 containing supernatant was bound to an anti-human Fab affinity column preequilibrated with PBS buffer, pH 7.0, on a FPLC (ÃKTA; Amersham Biosciences, Cardiff, U.K.). Fab59 was then eluted with 0.2 M glycine·HCl, pH 2.7, and immediately neutralized to pH 7.0 with 2 M Tris·HCl, pH 9.0. The neutralized eluate was dialyzed against TBS buffer, pH 7.0, overnight. After dialysis, the Fab59 sample was concentrated down to ≈1 ml and loaded to a size exclusion chromatography (SEC) column (Superdex 75, Amersham Biosciences; 10 × 110 cm) preequilibrated with TBS buffer, pH 7.0. SEC was performed on the FPLC with a flow rate at 0.4 ml per min. Monomeric Fab59 fractions were collected. Bacterial endotoxins were removed from Fab59 by passing through a Sartobind Membrane Adsorber Q15 (Sartorius, Elk Grove, IL). The sample was then concentrated by Centriprep YM-30 (Millipore, Billerica, MA) and filter-sterilized by Minisart-Plus (Sartorius). This nonoptimized protocol gave ≈3 mg per fermentor run. Optimization of growth and purification procedures continues to greatly improve the yield (unpublished data).
In Vivo Modeling of Fab59.
Male BALB/c mice, 9 weeks of age, were purchased from Taconic Farms (Germantown, NY) and maintained in animal facilities at Alexion Antibody Technologies. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Alexion. Mice were treated s.c. with Fab59 (5, 2, or 0.2 mg/kg twice per day), rhTPO made and purified in house (90 μg/kg, twice per day), or negative control antibody anti-TT Fab (5 mg/kg, twice per day) for 5 consecutive days. At different time points, peripheral blood was obtained from retroorbital plexus of animals by using heparinized Pasteur pipettes and immediately transferred to heparin-coated microcentrifuge tubes. The platelet counts were performed with a CELL-DYN 3700 counter (Abbott, Abbott Park, IL). Murine sera were obtained on days 15 and 29 for analysis of the immune responses in a direct ELISA.
ELISA for Antibody Response Against Human Fab, rhTPO, and rmTPO.
Nunc-Immuno plates (Nalge Nunc, Rochester, NY) were coated with 100 μl per well of Fab59, rhTPO, or rmTPO at 5 μg/ml in PBS and incubated at 4°C overnight. The plates were washed with 0.05% Tween 20 in PBS and then incubated with 200 μl of 1% BSA in PBS at RT for 1 h. After washing with 0.05% Tween 20 in PBS, the plates were incubated with 50 μl per well of serum samples (at a 1:10 dilution) at RT for 2 h. After washing, the plates were incubated with 100 μl per well of sheep anti-mouse IgG conjugated with peroxidase and absorbed with human serum proteins (Sigma, St. Louis, MO) at RT for 1 h, followed by incubation with 50 μl per well of tetramethylbenzidine (USB, Cleveland, OH) as substrate. The OD value was detected at a wavelength of 450 nm with a microplate reader.
Cloning and Expression of rhTPO for in Vivo Use.
Forward PCR primer (5′-CTCGGATCCGGCCGCCACCATGGAGCTGACTGAATTG-3′) and reverse primer (5′-TTCTAGAGTCAATGGTGATGGTGATGGTGCCCTTCCTGAGACAGATTCTG-3′) designed from the human TPO sequence [National Center for Biotechnology Information (NCBI)-AC:NM_000460] were used to amplify the TPO ORF from human liver Quick-Clone cDNA (Clontech, Palo Alto, CA). A BamHI site in the forward primer and an XbaI site in the reverse primer were introduced to subclone the PCR fragment into an Apex3P expression vector (developed by Alexion Pharmaceuticals). To optimize protein expression and facilitate purification, a Kozak sequence (GGCCGCCACCATGG) was included in the forward primer, and six histidines followed by a stop codon were inserted into the reverse primer. PCR was performed with the Expand High Fidelity PCR System (Roche Applied Science, Indianapolis, IN) by using procedures recommended by the manufacturer. The construct Apex3PhTPO-HIS was confirmed as correct by sequencing.
293 EBNA HEK cells were transfected with Apex3PhTPO-HIS by Effectene (Qiagen) and maintained in DMEM (Cellgro; Mediatech, Herndon, VA) at 37°C with 10% heat-inactivated FBS/2 mM glutamine/penicillin at 100 units per ml/streptomycin at 100 mg/ml/G418 sulfate at 250 μg/ml/puromycin at 1 μg/ml. After washing with HBSS (or PBS with Ca2+/Mg2+), cells were cultured in IS Pro serum-free medium (Irvine Scientific, Santa Ana, CA) supplemented with l-glutamine and penicillin/streptomycin. rhTPO in the supernatant was purified with a nickel-charged column (Ni-NTA, Qiagen).
Supplementary Material
Abbreviations
- TPO
thrombopoietin
- rhTPO
recombinant human TPO
- rmTPO
recombinant murine TPO
- cMpl-R
cMpl receptor
- CDR
complimentarity-determining region
- TT
tetanus toxoid
- HC
heavy chain
- LC
light chain
- EBNA
Epstein–Barr virus-encoded nuclear antigen
- RT
room temperature
Footnotes
References
- 1.McCullough J. Semin Hematol. 2000;37:3–10. doi: 10.1016/s0037-1963(00)90047-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuter DJ, Begley CG. Blood. 2002;100:3457–3469. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 3.Begley CG, Basser RL. Semin Hematol. 2000;37:19–27. doi: 10.1016/s0037-1963(00)90049-0. [DOI] [PubMed] [Google Scholar]
- 4.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, Davis AM, Tansik RL, Mattheakis LC, Boytos CM, et al. Science. 1997;276:1696–1699. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 5.Kratz-Albers K, Scheding S, Mohle R, Buhring HJ, Baum CM, McKearn JP, Buchner T, Kanz L, Brugger B. Exp Hematol. 2000;28:335–346. doi: 10.1016/s0301-472x(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T, Kaburaki H, Miyamoto S, Katayama J, Watanabe Y. J Biochem. 1997;122:1046–1051. doi: 10.1093/oxfordjournals.jbchem.a021845. [DOI] [PubMed] [Google Scholar]
- 7.Broudy VC, Lin NL. Cytokine. 2004;25:52–60. doi: 10.1016/j.cyto.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Nichol JL, Sullivan JT. Clin Pharmacol Ther. 2004;76:628–638. doi: 10.1016/j.clpt.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Orita T, Tsunoda H, Yabuta N, Nakano K, Yoshino T, Hirata Y, Ohtomo T, Nezu J, Sakumoto H, Ono K, et al. Blood. 2005;105:562–566. doi: 10.1182/blood-2004-04-1482. [DOI] [PubMed] [Google Scholar]
- 10.Erickson-Miller CL, DeLorme E, Tian S, Hopson CB, Stark K, Giampa L, Valoret EI, Duffy KJ, Luengo JL, Rosen J, et al. Exp Hematol. 2005;33:85–93. doi: 10.1016/j.exphem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Schwertschlag U, Turner KJ, Schendel P. In: Platelets. Michelson AD, editor. San Diego: Academic; 2002. pp. 845–854. [Google Scholar]
- 12.Kuter DJ. Transfusion. 2002;42:279–283. doi: 10.1046/j.1537-2995.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 13.Harker LA, Roskos LK, Marzec UM, Carter RA, Cherry JK, Sundell B, Cheung EN, Terry D, Sheridan W. Blood. 2000;95:2514–2522. [PubMed] [Google Scholar]
- 14.Vadhan-Raj S, Murray LJ, Bueso-Ramos C, Patel S, Reddy SP, Hoots WK, Johnston T, Papadopolous NE, Hittelman WN, Johnston DA, et al. Ann Intern Med. 1997;126:673–681. doi: 10.7326/0003-4819-126-9-199705010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 16.Vadhan-Raj S, Verschraegen CF, Bueso-Ramos C, Broxmeyer HE, Kudelka AP, Freedman RS, Edwards CL, Gershenson D, Jones D, Ashby M, et al. Ann Intern Med. 2000;132:364–368. doi: 10.7326/0003-4819-132-5-200003070-00005. [DOI] [PubMed] [Google Scholar]
- 17.De Serres M, Ellis B, Dillberger JE, Rudolph SK, Hutchins JT, Boytos CM, Weigl DL, DePrince RB. Stem Cells. 1999;17:203–209. doi: 10.1002/stem.170203. [DOI] [PubMed] [Google Scholar]
- 18.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5th Ed. Bethesda: US Dept of Health and Human Services; 1991. [Google Scholar]
- 19.Barbas CF, III, Kang AS, Lerner RA, Benkovic SJ. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbas CF, III, Rosenblum JS, Lerner RA. Proc Natl Acad Sci USA. 1993;90:6385–6389. doi: 10.1073/pnas.90.14.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbas SM, Ghazal P, Barbas CF, III, Burton DR. J Am Chem Soc. 1994;116:2161–2162. [Google Scholar]
- 22.Barbas CF, III, Burton DR, Scott JK, Silverman GJ. Phage Display: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. pp. 2.9–2.13.pp. 10.1–10.20. [Google Scholar]
- 23.Feese MD, Tamada T, Kato Y, Maeda Y, Hirose M, Matsukura Y, Shigematsu H, Muto T, Matsumoto A, Watarai H, et al. Proc Natl Acad Sci USA. 2004;101:1816–1821. doi: 10.1073/pnas.0308530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 25.Dorsch M, Fan PD, Danial NN, Rothman PB, Goff SP. J Exp Med. 1997;186:1947–1955. doi: 10.1084/jem.186.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbas CF, III, Languino LR, Smith JW. Proc Natl Acad Sci USA. 1993;90:10003–10007. [Google Scholar]
- 27.Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, Johnson DL, Barrett RW, Jolliffe LK, Dower WJ, et al. Science. 1996;273:458–463. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 28.Evans AJ, Krentz AJ. Drugs RD. 1999;2:75–94. doi: 10.2165/00126839-199902020-00001. [DOI] [PubMed] [Google Scholar]
- 29.Verma IM, Deschamps J, Van Beveren C, Sassone-Corsi P. Cold Spring Harbor Symp Quant Biol L. 1986;1:949–958. doi: 10.1101/sqb.1986.051.01.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.