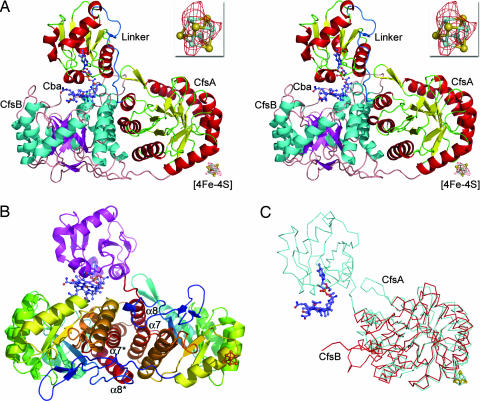
Fig. 1.
Overall structure of CoFeSPCh from C. hydrogenoformans. (A) Stereo ribbon presentation of the CoFeSPCh structure. CfsA is shown with α-helices in red and β-sheets in yellow, and CfsB is shown with α-helices in cyan and β-sheets in magenta. Cba denotes the cobamide cofactor. The proline-rich linker connecting the middle and C-terminal domains of CfsA is blue and labeled as linker. Cofactors (corrinoid cofactor and [4Fe-4S] center) are presented as a ball-and-stick model (Co in wheat, C in light blue, O in red, N in dark blue, Fe in aquamarine, and S in dark yellow). (Inset) An anomalous difference Fourier map (red) around the [4Fe-4S] cluster, contoured at 3.0σ, is shown with the overall structure and magnified. (B) Ribbon presentation of CfsA and CfsB with the (βα)8-barrel domains in rainbow colors from the N termini in blue to the C termini in red. The C-terminal domain of CfsA is shown in magenta. Helices forming the four-helical bundle like arrangement in the subunit interface are labeled; a star denotes helices of CfsB. (C) Superimposition of CfsB (red) on CfsA (aquamarine). All pictures were prepared by using PyMol (26).