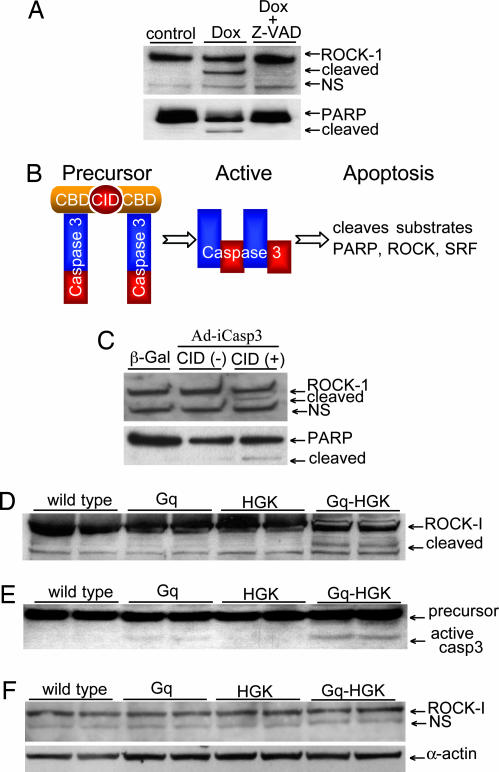
Fig. 2.
ROCK-1 cleaved by caspase-3 in apoptotic cardiomyocytes and in transgenic heart failure mouse models. (A) A Western blot of whole-cell lysates from controls and doxorubicin-treated (Dox) neonatal rat cardiomyocytes revealed enhanced ROCK-1 and PARP cleavage. Caspase-3 inhibitor Z-VAD-fmk blocked caspase-3 cleavage. (B) A schematic diagram of a dimerizer inducible activation system for caspase-3. An adenovirus expressed a caspase-3 precursor fused to a modified FK506-binding domain, and a chemical inducer binding domain. Dimerization of caspase-3 precursors via CID forces caspase-3 activation and rapid cellular apoptosis. (C) A Western blot of whole-cell lysates from neonatal rat cardiomyocytes showed intact ROCK-1 after caspase-3 adenovirus infection. The addition of CID led to ROCK-1 cleavage shown by the appearance of the 130-kDa species. Control adenovirus express β-gal. (D and E). To assess ROCK-1 cleavage in vivo, hearts from Gq, HGK, and bigenic Gq-HGK mice were assessed by Western blot analysis for ROCK-1 and caspase-3 activity. A 130-kDa fragment of ROCK-1 was observed only in hearts from bitransgenic mice paralleled with a significant increase in caspase-3 activity. (F) The same heart specimens assessed by a specific antibody against C-terminal ROCK-1 revealed only a full-length species of ROCK-1.