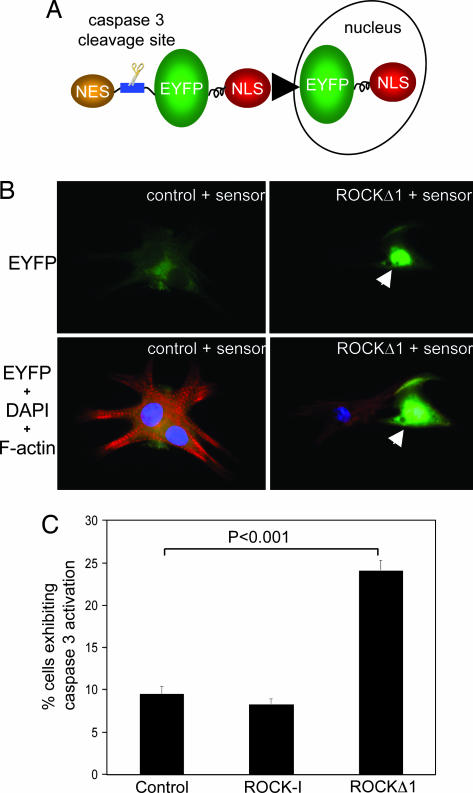
Fig. 3.
Activated mutant, ROCKΔ1, induced caspase-3 activation and apoptosis in neonatal rat cardiomyocytes. (A) Schematic diagram of a caspase-3 sensor, which incorporated a caspase-3-specific cleavage site located between EYFP and NES. When caspase-3 is inactive, the dominant NES targets EYFP to the cytosol. After the induction of apoptosis, the export signal is removed by active caspase-3, which triggers the redistribution of EYFP from cytosol to the nucleus via NLS (nuclear localization signal). (B) Representative images showed cardiac myocytes subjected to ROCKΔ1 accumulated EYFP in myocyte nuclei indicated by arrows and developed apoptosis, as shown by disorganized myofilaments. Green, EYFP-fusion protein; red, rhodamine-conjugated phalloidin staining for F-actin; blue, DAPI nucleus staining. (C) The prevalence of caspase-3 activation was evaluated by the percentage of transfected cardiomyocytes exhibiting caspase-3 activation (nuclear localization of EYFP-fusion protein). Results are the average ± standard error of four separate experiments.