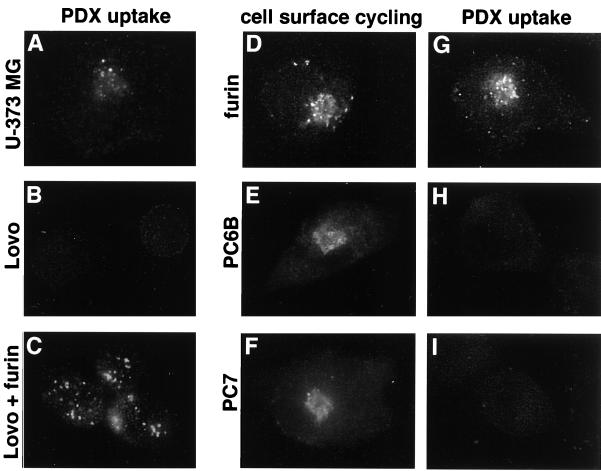
Figure 4.
Uptake of α1-PDX requires cellular furin. (A–C) Parallel coverslips of U-373 MG (A) and LoVo (B and C) cells either noninfected (A and B) or infected with a vaccinia recombinant expressing nontagged furin (moi = 10) for 4 h (C) were incubated with 8 μM α1-PDX (FLAG-tagged) for 1 h before fixation and staining with an anti-FLAG mAb (mAb M2) to detect internalized α1-PDX. LoVo cells infected with wild-type vaccinia virus showed no uptake of α1-PDX (data not shown). (D–I) U-373 MG cells on replicate coverslips were infected with vaccinia recombinants expressing epitope (FLAG)-tagged furin (D and G), epitope (FLAG)-tagged PC6B (E and H), or epitope (FLAG)-tagged PC7 (F and I) for 4 h. The cultures were then incubated with an anti-FLAG mAb (mAb M1) for 1 h (D–F) to demonstrate cell-surface cycling of each FLAG-tagged convertase or with α1-PDX (G–I). The cells were fixed, permeabilized, and analyzed by immunofluorescence microscopy. Internalized mAb M1 was detected with a goat anti-mouse IgG2b-TxRD. Internalized α1-PDX was detected with an anti-α1-anti-trypsin antiserum followed by goat anti-rabbit-FITC. The lower immunofluorescent signal detected in H and I compared with A (i.e., PDX uptake by endogenous furin) was caused by lower sensitivity of the α1-anti-trypsin antiserum relative to mAb M2.