Abstract
Evidence from in vitro studies indicates that complement activation regulates the expression of P-selectin on endothelial cells. This suggests that in disorders such as ischemia/reperfusion injury, in which both complement and P-selectin have been shown to play a role, complement activation is a primary event and the effects of P-selectin are secondary. To test this hypothesis in vivo, we examined a mouse kidney model of ischemia/reperfusion injury. Surprisingly, the time course and extent of expression of P-selectin was unaltered in C3-deficient mice compared with wild-type mice, in which there was rapid but transient up-regulation of P-selectin on capillary walls and slower accumulation of complement split product on the tubular epithelium. In addition, treatment with anti-P-selectin antibody to reduce the neutrophil-mediated reperfusion damage was equally effective in the absence of C3. These data imply that complement and P-selectin-mediated pathways of renal reperfusion injury are mutually independent, a conclusion that is possibly explained by the differences in the location and time kinetics of complement activation and P-selectin expression. We conclude that in vivo interaction between complement and P-selectin is limited because of time and spatial considerations. Consequently, complement and P-selectin pose distinct targets for therapy.
Reperfusion injury after a period of ischemia1 involves the development of endothelial and parenchymal damage, neutrophil infiltration, as well as increased expression of activated complement components2 and dysregulation of adhesion molecules3 and cytokines.4,5 The clinical outcome in conditions such as stroke, myocardial infarction, acute renal failure, and solid organ transplantation depends on the severity of the ischemia/reperfusion (I/R) injury. The pathophysiology is complex and thought to involve at least three major contributory factors: activated neutrophils, which cause tissue damage by releasing cytotoxic proteases and oxygen-derived radicals; complement; and adhesion molecules.6–8
Although complement activation and neutrophil infiltration have been shown to be important in the setting of renal ischemia, as in other organs, the principal order of involvement of these components is unclear. The currently accepted paradigm of complement and neutrophil involvement in the inflammatory response suggests that the earliest step is activation of the complement cascade.9 Activation of complement releases a number of proinflammatory mediators, of which C5a is a potent chemoattractant that can directly mediate chemotaxis and activation of neutrophils,10 resulting in tissue injury. Neutrophil recruitment is also dependent on the action of adhesion molecules,11 in particular P-selectin, a 140-kd membrane glycoprotein of platelet and endothelial cell granules. After activation of endothelium, preformed P-selectin is expressed on the cell surface, a process that is rapid and can occur within 1 minute of endothelial activation.12 P-selectin is responsible for the initial tethering13 and rolling14 of neutrophils on the surface of activated microvascular endothelium. Notably, recent studies in mouse have demonstrated that blockade of P-selectin conferred protection from ischemic injury after reperfusion.15,16
It is known from in vitro studies that C5a-stimulated human umbilical vein endothelial cells up-regulate P-selectin, which are then able to bind nonstimulated neutrophils,17 and that neutrophils adhere strongly to P-selectin-coated plates.18 This evidence points to a direct causal link between activated complement, P-selectin expression, and neutrophil recruitment. Other in vitro studies have shown that the terminal membrane attack complex (MAC) causes rapid surface expression of P-selectin on cultured endothelial cells.19 In another study, however, sublytic amounts of MAC were shown to up-regulate expression of adhesion molecules such as endothelial leukocyte adhesion molecule-1, but had no effect on P-selectin.20 These studies have highlighted interactions between all three major components, which makes it difficult to understand the extent to which the respective mechanisms interact in vivo, be they mutually dependent, or whether they constitute separate pathways of injury. Such understanding is crucial to the development of effective therapy.
Previous work in complement-deficient mice showed a significant sparing of renal I/R injury, including a 40% reduction of infiltrating neutrophils.21 As it is now accepted that P-selectin expression is a prerequisite for the initial adhesion of neutrophils to activated endothelium, and that complement may also have a bearing on neutrophil activity at sites of inflammation, we reasoned that determining the activity of P-selectin in complement-deficient mice would help to identify the interaction between these two systems. If P-selectin activity were reduced in the absence of functional complement, this would suggest a role for complement in the regulation of P-selectin expression. On the other hand, if P-selectin activity were unabated in the absence of complement activation, this would suggest that P-selectin expression was independent of complement.
Here we report evidence that P-selectin and complement contribute separately to renal injury after I/R, and propose that differences in the site and timing of the P-selectin and complement activities may explain their lack of interaction in vivo.
Materials and Methods
Animals
Homozygous C3-deficient mice were derived as described previously.22,23 As C3-deficient mice were produced using C57BL/6 (B6) parents with SV129 embryonic stem cells, progeny were backcrossed onto the B6 parental strain for a minimum of six generations. Progeny were maintained in specific pathogen-free conditions. Wild-type B6 controls were purchased from Harlan UK Ltd. (Bicester, UK). Male mice were used throughout the experiments. All procedures for animal work were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986.
Ischemia Protocol
The renal ischemia protocol has been described previously.21 Briefly, Mice, weighing 22 to 25 g were anesthetized by inhalation of Enflurane and by intraperitoneal administration of Hypnovel (6.64 mg/kg), to reduce usage of Enflurane (Abbott Laboratories Ltd, Kent, UK). We preferred this form of anesthesia because it proved less toxic than barbiturate and allowed more reproducible I/R injury. The mortality through the whole study was 2 to 3%. Body temperature was kept constant by placing a warm pad beneath the animal. Using a midline abdominal incision, renal arteries and veins were bilaterally occluded for 55 minutes with microaneurysm clamps (Codman, Berkshire, UK). This period of ischemia was predetermined based on a previous study in this laboratory.21 After removal of the clamps, 0.4 ml of warm saline was placed in the abdomen, the incision was sutured, and animals were returned to their cages. Tail blood samples were taken at 24 hours after removing the clamps, and at 48 hours the mice were sacrificed. Terminal blood samples were collected by cardiac puncture and kidneys were harvested for morphological analysis. Groups of additional mice were performed identically and sacrificed at 5, 15, 30, and 60 minutes after reperfusion, whereupon kidneys were harvested for immunochemical staining and blood samples obtained by cardiac puncture for assessment of renal function.
P-Selectin Blockade and Neutrophil Depletion
P-selectin experiments used a function-blocking rat anti-mouse P-selectin antibody RB40.34, or isotype-matched control antibody A110.1 (BD Pharmingen, San Diego, CA). For our ischemia studies, groups of wild-type (WT) and complement-deficient mice were given a 40-μg dose of RB40.34 or A110.1 by intraperitoneal injection and 4 hours after antibody treatment, animals were subjected to bilateral renal ischemia as described above. The dose of antibody (RB40.34) was predetermined by the inhibition of thioglycollate-mediated migration of polymorphonuclear neutrophils into the peritoneum of WT mice, using a range of concentrations from 5 to 100 μg administered intraperitoneally to WT mice. We found that 40 μg was the optimal dose for inhibiting polymorphonuclear neutrophil recruitment to the peritoneum. In a further study we confirmed that functioning RB40.34 is efficacious at 6 hours after intraperitoneal administration. WT mice were administered a 40- or 25-μg dose of RB40.34 by intraperitoneal injection and serum was obtained 6 hours later. Harvested serum was then administered intraperitoneally to WT mice and its ability to block thioglycollate-induced peritoneal migration of polymorphonuclear neutrophils was determined. We found that transferred serum had sufficient anti-P-selectin activity to completely block polymorphonuclear neutrophil migration into the peritoneum at both concentrations. In other experiments, WT or complement-deficient mice were administered a 150-μl dose of rabbit anti-mouse neutrophil serum (ANS; Intercell Technologies, Hopewell, NJ), or control normal rabbit serum (DAKO Ltd., Cambridge, UK), together with a 40-μg dose of either anti-P-selectin monoclonal antibody (mAb) or isotype-matched control mAb per animal by intraperitoneal injection. Four hours after administration of antibody and ANS, animals were subjected to 55 minutes of bilateral renal ischemia, as previously described. Normal rabbit serum used above was dialyzed against phosphate-buffered saline (PBS) to remove sodium azide. The dose of ANS and control normal rabbit serum was chosen on the basis of a previous study.24 The efficacy of neutrophil depletion was confirmed by counting circulating neutrophils in treated animals 4 hours after ANS treatment.
Inhibition of Complement Activity and P-Selectin Blockade
In this experiment, 1 mg of monoclonal anti-mouse C5 antibody (BB5.1) was injected intraperitoneally 16 hours before induction of renal ischemia, in the presence or absence of 40 μg of anti-P-selectin mAb, provided intraperitoneally 4 hours before induction of ischemia. The efficacy of inhibition of complement activity by anti-mouse C5 antibody was confirmed by measuring hemolytic activity in animals treated with this antibody as described previously.21,25
Assessment of Renal Function
Blood urea nitrogen (BUN) was determined using a standard urease assay kit from Sigma [BUN (Infinity); Sigma-Aldrich Co., Dorset, UK]. We have previously found excellent correlation between BUN and serum creatinine measurements in this setting (correlation coefficient r2 = 0.8675).26
Assessment of Renal Pathology
Kidney removed surgically at specified times after ischemia, was cut coronally and fixed in a solution of 1% paraformaldehyde for 24 hours and embedded in paraffin. Sections (2 μm) were stained using the periodic acid-Schiff reaction and reviewed in a blinded manner by two persons. From four coronal sections per animal, at a magnification of ×160, the percentage of tubules in the corticomedullary junction showing epithelial necrosis was estimated, using a well-established five point scale: 0, normal kidney; 1, less than 10% necrosis; 2, 10 to 25% necrosis; 3, 26 to 75% necrosis; and 4, greater than 75% necrosis.24 To detect the presence of thrombosis within kidney sections, Martius scarlet and blue staining was used for highly selective detection of thrombosis.27
Immunochemical Staining
Coronally cut kidney (half kidney) was embedded in OCT compound and frozen in liquid nitrogen. Frozen sections (4 μm) were air-dried for a minimum of 20 minutes at room temperature and then acetone-fixed. Sections were incubated with rat anti-mouse P-selectin clone RB40.34, followed by biotin-conjugated secondary antibody and finally by streptavidin-peroxidase conjugate. For neutrophil staining, rat anti-mouse neutrophil antibody (Serotec, Oxford, UK) was followed by horseradish peroxidase-conjugated goat anti-rat IgG. For C3 staining, rabbit anti-human C3d was followed by fluorescein isothiocyanate-conjugated goat anti-rabbit IgG. The primary antibody for C3d staining, all secondary reagents and streptavidin-peroxidase conjugate were purchased from DAKO, Cambridge, UK. The amount of complement deposition and P-selectin expression were quantified using LUCIA image analysis software (Jencons-PLS, Forest Row, East Sussex, UK). Briefly, at a magnification of ×400, for each animal, 20 fields from two stained kidney sections were photographed. Areas of positive staining in each image were outlined, highlighted, and calculated automatically.
Neutrophil Counts and Myeloperoxidase (MPO) Activity
After immunochemical staining for neutrophil infiltration as described above, discrete, individually stained neutrophils were counted within the corticomedullary junction using an eyepiece graticule at a magnification of ×250 and expressed as number of neutrophils per field. For each animal, 10 fields from four kidney sections were counted. MPO activity, used as an indicator of neutrophil infiltration, was measured as previously described.28,29 Harvested kidney tissue (quarter kidney), was homogenized in 5 mmol/L of potassium phosphate buffer (pH 6.0). Homogenates were centrifuged at 30,000 × g for 20 minutes at 4°C. The pellets were resuspended in 1 ml of extraction buffer (50 mmol/L potassium-phosphate buffer, pH 6.0, containing 0.5% hexadecyl trimethylammonium bromide) and then subjected to three rounds of freeze-thawing. Supernatants were generated by clarification at 13,000 × g for 15 minutes at 4°C. Sample protein concentrations were determined using a Micro BCA protein assay reagent kit (Pierce Chemical Co., Rockford, IL), using bovine serum albumin for a standard curve. MPO was assayed using a microtiter plate method, which involved mixing 100 μl of sample with 100 μl of reaction buffer (50 mmol/L potassium-phosphate buffer, pH 6.0, containing 0.167 mg/ml of O-dianisidine dihydrochloride and 0.0006% H2O2). Absorbance was measured at 460 nm and MPO content was converted to U of MPO activity per mg of protein and represented as percentage MPO activity above that of normal mouse kidney.
Intraperitoneal Neutrophil Migration
To determine the intrinsic ability of neutrophils to migrate to sites of inflammation in C3-deficient mice, thioglycollate, an effective inducer of neutrophil-mediated inflammation was used as described previously.30 Because the action of thioglycollate is dependent on the activity of the alternative pathway of complement activation, C3-deficient mice and WT controls were administered 150 μl of WT serum intraperitoneally as a source of C3. Control WT and C3-deficient mice were injected with PBS. After 45 minutes, all mice were injected intraperitoneally with 1 ml of 3% thioglycollate (Difco, Detroit, MI) in PBS. After 3 hours, mice were sacrificed and the peritoneal cavity was washed with 3 ml of PBS three times. Total cells from the lavage were recovered by centrifugation at 350 × g. Red blood cells were lysed by resuspending in 0.5 ml of water for 15 seconds. Isotonicity was restored by adding 30 ml of PBS. Contaminating macrophages were removed by adherence to nontissue culture-grade Petri dishes for 30 minutes at room temperature. Unbound neutrophils were recovered and counted using a hemocytometer and subpopulations were determined by differential counts on hematoxylin-stained smears.
Statistical Analysis
Results are expressed as arithmetic means (± SEM). Statistical analyses between experimental groups were performed using unpaired Student’s t-test. A difference was considered to be significant when P < 0.05.
Results
P-Selectin and Activated Complement Are Expressed at Different Sites and Different Times after Reperfusion in Ischemic Kidney
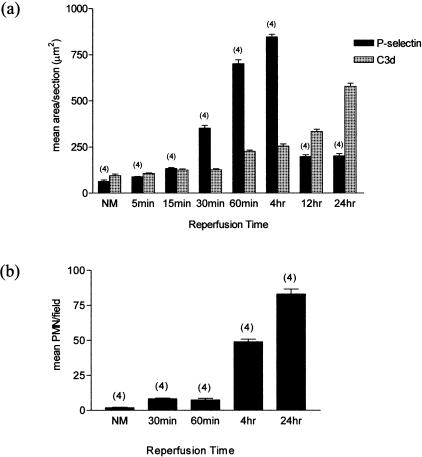
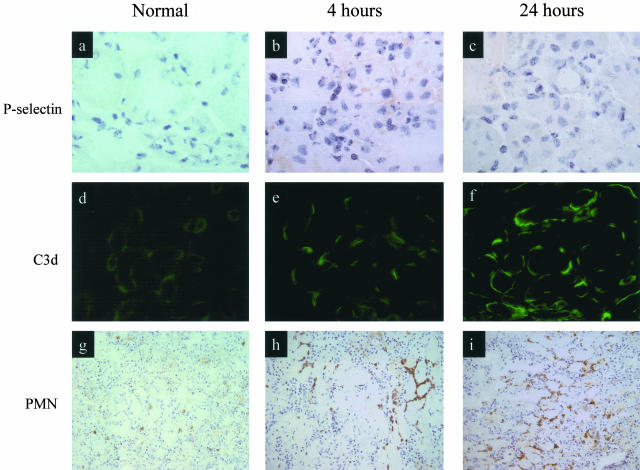
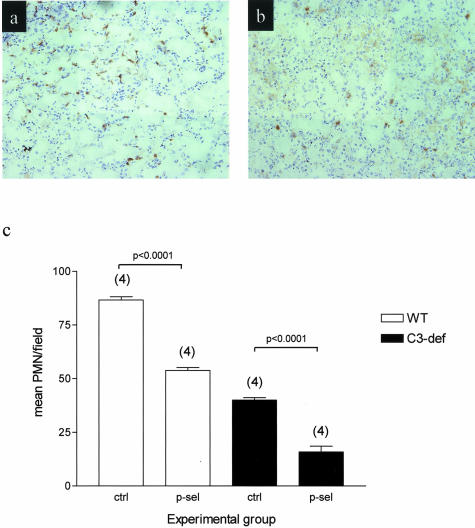
We performed detailed examination of P-selectin expression and C3 split product deposition in the first 24 hours after the reperfusion of ischemic mouse kidney. Quantitative image analysis of stained tissues showed that P-selectin expression was rapidly up-regulated, with peak expression by 4 hours after reperfusion (Figure 1a and Figure 2, b and c). In contrast, peak deposition of C3 split product occurred at least 24 hours after reperfusion, by which time P-selectin expression had almost returned to baseline (Figure 1a and Figure 2, e and f). Up-regulation of P-selectin occurred only on the capillary wall, whereas C3 deposit (and MAC; not shown) was present mainly on the surface of damaged tubules (Figure 2, e and f). Neutrophil infiltration peaked at 24 hours after reperfusion, primarily involving the interstitial areas around damaged tubules (Figure 2, h and i).
Figure 1.
Effect of ischemia (55 minutes) and reperfusion on P-selectin expression, complement deposition, and neutrophil infiltration in WT mice. a: Relationship between P-selectin and complement at different times after reperfusion. In each group, mean areas of positive P-selectin expression or complement deposition were determined within 40 high-powered fields per animal (see Materials and Methods). b: Infiltration of neutrophils at different stages after reperfusion. In each group, mean neutrophil counts were determined in 8 to 12 fields per animal. Numbers in parentheses represent numbers of animals studied at each point.
Figure 2.
Staining for P-selectin expression, complement activation product, and neutrophil infiltration. Normal untreated WT mice and WT mice treated with 55 minutes of bilateral renal ischemia followed by reperfusion for 4 and 24 hours. a, b, and c: P-selectin expression is seen on corticomedullary small peritubular vessels. d, e, and f: Immunochemical staining of C3d is present on the basolateral surface of renal tubular epithelium. g, h, and i: Immunochemical staining of neutrophils in the corticomedullary junction. Original magnifications: ×1000 (a–c); ×400 (d–f); ×250 (g–i).
P-Selectin Expression Is Unimpaired in C3-Deficient Mice
We next examined C3-deficient mice to determine whether up-regulation of renal P-selectin was altered in the absence of complement activation. P-selectin expression after 30 minutes of reperfusion was not significantly different from that in WT mice (mean area of P-selectin expression/section 80.9 ± 9.3 μm2 versus 90.0 ± 4.63 μm2; P > 0.05). Similarly, after 4 hours of reperfusion there was no significant difference in P-selectin expression between C3-deficient and WT mice (315.5 ± 59.1 μm2 versus 225.9 ± 31.7 μm2; P > 0.05). Thus, dysregulation of P-selectin occurred in the absence of complement activation.
P-Selectin Blockade Is Effective in the Absence of Complement Activation
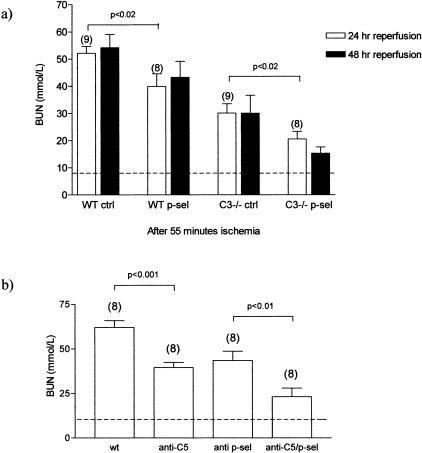
To examine the function of P-selectin in the absence of complement activation, we induced I/R injury in C3-deficient or WT mice after pretreatment with anti-P-selectin antibody or control antibody. Whereas the combined effect of C3 deficiency and P-selectin blockade was to reduce renal functional impairment by 62 to 73%, C3 deficiency alone led to 46 to 52% reduction, and P-selectin inhibition to 27 to 28% reduction, compared with their respective controls (Figure 3a). Thus, P-selectin blockade was effective even in the absence of complement activation. Furthermore, the effects of C3 deficiency and P-selectin inhibition were additive, implying that P-selectin exerts an effect on renal functional injury independently of complement. Structural damage, consisting of thinning and degeneration and shedding of the tubular epithelium, with tubule cast formation, was present only in the hypoxia-sensitive region of the corticomedullary junction. The amount of damage was proportional to the degree of functional impairment in the respective groups of mice (Table 1 and Figure 4).
Figure 3.
Effect of renal ischemia on renal function in WT, C3-deficient, and complement-inhibited mice. a: WT and C3-deficient mice underwent 55 minutes of bilateral renal ischemia 4 hours after receiving anti-P-selectin mAb or isotype-matched control mAb, as indicated. Blood urea nitrogen was measured 24 to 48 hours after removal of the clamps. b: WT mice were complement-inhibited by intraperitoneal administration of anti-C5 mAb 16 hours before induction of ischemia. WT and anti-C5-treated WT mice underwent 55 minutes of bilateral renal ischemia 4 hours after receiving anti-P-selectin mAb as indicated. Reperfusion was for 24 hours. Dashed line represents BUN measurement in normal animals. Numbers in parentheses represent the number of animals studied in each group. P values are for comparisons as shown. P values of <0.05 were seen after 48 hours of reperfusion.
Table 1.
Severity Score of Renal Tubular Damage
| Mice | Score | P value |
|---|---|---|
| WT control-treated | 3.1 ± 0.96 (n = 9) | — |
| WT anti-P-selectin-treated | 2.4 ± 0.55 (n = 8) | <0.05 |
| C3-deficient control-treated | 2.0 ± 0.32 (n = 9) | <0.001 |
| C3-deficient, anti-P-selectin-treated | 1.3 ± 0.56 (n = 8) | <0.001 |
Values presented are means ± SEM. Numbers in parentheses represent the number of animals studies in each group. Between two and three sections were evaluated per animal. P values are for comparison of all groups to WT control-treated group.
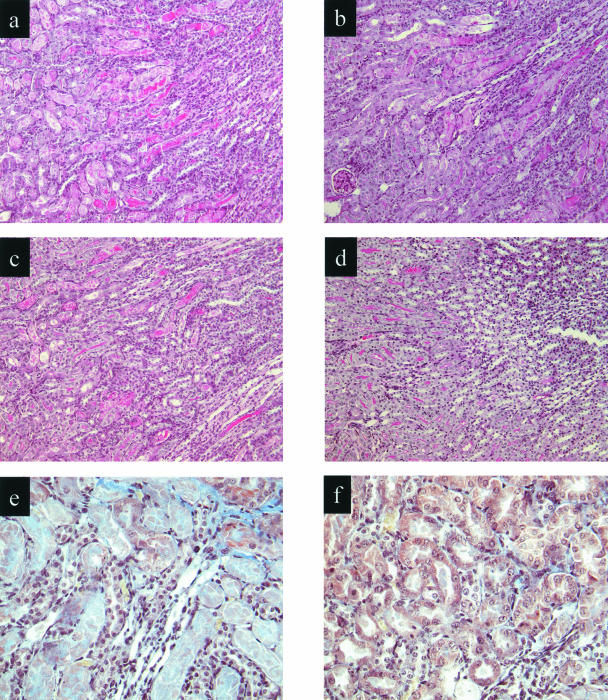
Figure 4.
Effect of renal ischemia and reperfusion on renal morphology and fibrin deposition in WT and C3-deficient mice pretreated with anti-P-selectin or control mAb. Light microscopy of kidney corticomedullary junction showing tubular damage at 48 hours of reperfusion. a: WT control-treated; b: WT anti-P-selectin-treated; c: C3-deficient control-treated; d: C3-deficient, anti-P-selectin-treated mice. e and f: Martius scarlet and blue staining at damaged tubular area in WT control-treated (e) and C3-deficient, anti-P-selectin-treated (f) mice. Fibrin, erythrocytes, nuclei, and connective tissue stain as red, yellow, black, and blue, respectively. Original magnifications: ×250 (a–d); ×400 (e, f).
To confirm these findings and show that the observations in C3-deficient mice were the result of complement deficiency and not an artifact of gene disruption, we performed studies in WT mice treated with complement inhibitor. For this we used an anti-mouse C5 mAb (BB5.1), because this antibody has been shown to block the formation of both C5a and C5b-9, which are thought to be the main complement effector products of mouse kidney I/R injury.21,31 Furthermore, a single intraperitoneal injection of BB5.1 results in marked inhibition of complement hemolytic activity for several days.25 We confirm here that treatment of WT mice with BB5.1 inhibited complement hemolytic activity for the full 24-hour period required for our study when compared to nontreated WT animals (20.55% ± 1.35 versus 83.05% ± 0.65; P < 0.05). Under these conditions, renal functional impairment in the presence of combined complement and P-selectin blockade was reduced by 63% compared with only 31% with P-selectin treatment alone (Figure 3b). Anti-P-selectin therapy was therefore effective in complement-inhibited as well as in complement-deficient mice.
P-Selectin Blockade Is Sufficient to Explain the Effects of Neutrophil-Mediated I/R Injury
As illustrated in Figure 5, both complement and P-selectin can mediate inflammatory cell infiltration into postischemic kidney, as assessed by neutrophil-specific immunohistochemistry and neutrophil counts. Neutrophil infiltration was compromised furthest in C3-deficient mice treated with anti-P-selectin antibody, when compared with C3 deficiency alone, or with P-selectin inhibition alone (Figure 5c). The reduction in neutrophil infiltration was proportional to the degree of functional impairment in the respective experimental groups (Figure 3 and Figure 5c). Tissue MPO activity measurement was in agreement with neutrophil counts, ie, anti-P-selectin treatment reduced MPO activity in WT mice (83.7% ± 24.6 versus 142.6% ± 28.8) and C3-deficient mice (23.6% ± 8.5 versus 43.7% ± 18.2), compared to control antibody treatment. MPO activity is expressed as percentage of normal nonischemic kidney. Residual MPO activity was observed in both groups and may represent expression by other infiltrating cells such as macrophages.
Figure 5.
Neutrophil infiltration in WT and C3-deficient mice after I/R insult. Mice underwent 55 minutes of bilateral ischemia 4 hours after receiving anti-P-selectin mAb or isotype-matched control mAb. Immunochemical staining of neutrophils at the corticomedullary junction after 48 hours of reperfusion in WT control-treated mice (a) and C3-deficient, anti-P-selectin-treated mice (b). c: Infiltration of neutrophils 48 hours after reperfusion. In each experimental group, mean neutrophil counts were determined in 8 to 12 fields per animal. Numbers in parentheses represent numbers of animals studied at each point. Original magnifications, ×250.
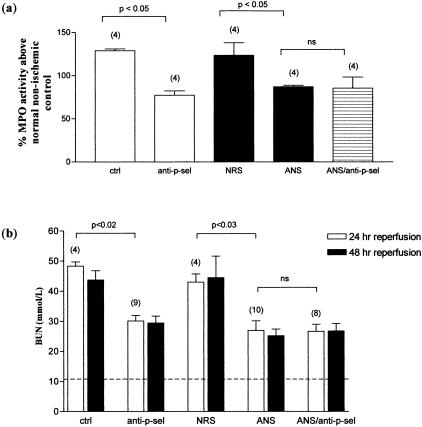
To clarify the efficiency of P-selectin blockade in preventing neutrophil incursion into injured tissue, we compared the effect of P-selectin blockade with a specific neutrophil-depleting antiserum. Neutrophil depletion 4 hours before the induction of ischemia was found to be effective because preliminary studies demonstrated a 78.3% reduction in the number of circulating neutrophils compared to control-treated and normal WT control animals. As can be seen in Figure 6, both MPO activity (Figure 6a) and functional renal injury (Figure 6b) were reduced after neutrophil-specific depletion or P-selectin blockade. Each treatment had a similar degree of effect. The effect of combined P-selectin and neutrophil inhibition was nonadditive, indicating complete overlap of function (Figure 6). Our preliminary studies showing complete arrest of neutrophil migration in the presence of P-selectin blockade (see Materials and Methods), suggest that anti-P-selectin antibody was an efficient means of preventing neutrophil migration into reperfused tissue. The remaining MPO activity after P-selectin blockade is therefore likely to be explained by a nonneutrophil source.
Figure 6.
Effect of neutrophil depletion and anti-P-selectin therapy after ischemia. WT mice underwent 55 minutes of bilateral renal ischemia 4 hours after receiving an injection of ANS or control normal rabbit serum (150 μl/mouse), in combination with anti-P-selectin mAb or isotype-matched control mAb (40 μg/mouse), as shown. a: MPO activity was measured in the kidney tissue after 48 hours of reperfusion. b: BUN was measured at 24 and 48 hours after removal of clamps. Dashed line represents BUN measurement in normal animals. Numbers in parentheses represent numbers of animals studied at each point. P values are for comparison of treated groups with relevant control-treated groups.
The above experiments show that C3-deficient mice had reduced neutrophil infiltration after I/R. To demonstrate that the defect in neutrophil infiltration was because of C3 deficiency and not to an intrinsic defect in neutrophil migration in the mutant mice, we assessed the capacity for neutrophil migration after intraperitoneal injection of thioglycollate. In C3-deficient mice reconstituted with whole serum, the neutrophil response was comparable with that achieved in WT control mice (total cell counts in the peritoneal washout: 6.91 × 106 ± 1.97 versus 4.94 × 106 ± 1.03). Neutrophil migration was severely compromised in C3-deficient mice that had not received whole serum as a means of restoring complement activity (total cell count in the peritoneal washout: 2.35 × 106 ± 1.17). WT mice injected with whole serum before challenge with thioglycollate elicited a neutrophil response comparable to WT controls that received PBS before thioglycollate, confirming that whole serum did not induce an indirect effect on the migration of neutrophils in WT animals (data not shown).
Discussion
In this study we have attempted to define the relationship between complement activation, P-selectin expression, and neutrophil recruitment in the setting of renal I/R injury. We anticipated that up-regulation of P-selectin would be reduced in complement-deficient mice, in view of the known ability of C5a and MAC to increase P-selectin expression on cultured endothelial cells.17,19 However, neither the expression nor the pathological function of P-selectin was affected in complement-deficient animals. Moreover the effects of C3 deficiency and P-selectin appeared to be additive in the prevention of renal dysfunction. We therefore propose that the underlying mechanisms of complement-mediated and P-selectin-mediated renal I/R injury are distinct.
The function of P-selectin is to mediate leukocyte emigration via the microvascular endothelium at sites of inflammation.32 Although many factors, eg, chemokines, leukotrienes, and integrins, can also contribute to neutrophil migration, P-selectin had a major effect in our model. The anti-inflammatory effect of P-selectin blockade was equal to the effect of specific neutrophil depletion in our experiments, both in functional and MPO studies. Furthermore, there was no additional benefit when neutrophil-depleting antibody was combined with P-selectin blockade. In other words, P-selectin activity accounted substantially for the migration of the neutrophil population defined by immunodepletion. Tissue neutrophil activity was not completely prevented by either treatment, and therefore blockade of neutrophil function in our studies could have been incomplete.
Previous work on C6-deficient mice has highlighted a role for the terminal components of complement in renal I/R injury, and suggested that chemoattractant factors released at earlier stages of complement activation are less important for the development of such injury.21 The data presented here are consistent with this view, in that P-selectin mediation was sufficient to account for the neutrophil effect in our model of renal I/R injury, whereas complement appeared to act separately and cause an additive effect on tissue injury. Other studies have identified a role for the anaphylatoxin C5a in both cardiac33 and renal I/R damage, although data in the renal model indicate that the effect of C5a may be mediated through action on the tubule cell, which bears C5a receptors, rather than a neutrophil-mediated effect.31
It is known that C5a and MAC can regulate the surface expression of P-selectin on cultured endothelial cells. In distinction, our present studies show that the expression and function of P-selectin was unmodified by the absence of complement activation in C3-deficient mice, and that anti-P-selectin treatment was effective even in the presence of C5 blockade. In explaining the lack of effect of complement on P-selectin in vivo, we note that P-selectin had an early wave of expression peaking at 4 hours after reperfusion and that P-selectin was mainly located on the capillary wall. In contrast, C3 deposition occurred predominantly on the renal tubule and exhibited a slower rate of accumulation. After reperfusion and subsequent activation of endothelium by the resulting metabolites of ischemia, preformed P-selectin translocates to the endothelial cell surface within minutes, from intracellular granules called Weibel-Palade bodies and from the α granules of platelets.34 In addition to this early wave of expression, P-selectin is also transcriptionally regulated and expression is transiently induced 2 to 4 hours later by lipopolysaccharide, tumor necrosis factor-α, and interleukin-1.34 Although complement activation is also a rapid process, the ongoing accumulation of complement split product is likely to represent amplification of the complement cascade by the alternative pathway, after an initial triggering event.35 It is highly possible therefore, that the peak activities of complement and P-selectin did not coincide in vivo, and hence failed to show significant interaction. Concurrent analysis of neutrophil infiltration shows a marked increase in numbers from 4 hours of reperfusion, coinciding with peak expression of transcriptionally induced P-selectin.36,37
In conclusion, our data suggest a particular order of events after renal ischemia and reperfusion. An initial burst of P-selectin expression is followed by P-selectin-mediated neutrophil infiltration and complement attack on the renal tubules. This order of events, as well as the different tissue-compartment locations of P-selectin and complement in post-ischemic mouse kidney, may explain the independence of P-selectin and complement in the functional inhibition studies. Furthermore, although anti-P-selectin antibody and neutrophil depletion are likely to be redundant as a therapeutic combination, dual therapy targeting P-selectin and the complement cascade (at intravascular and extravascular sites, respectively) may prove successful.
Footnotes
Address reprint requests to Dr. Wuding Zhou, Department of Nephrology and Transplantation, 5th Floor, Thomas Guy House, Guy’s Hospital, King’s College, London, SE1 9RT, UK. E-mail: wuding.zhou@kcl.ac.uk.
Supported by the Wellcome Trust (ref. 0044386) of the United Kingdom and the Medical Research Council.
References
- Grace PA. Ischemia-reperfusion injury. Br J Surg. 1994;81:637–647. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne MJ, Elghandour A, Haq M, Saba SR, Norman J, Condon T, Bennett F, Rabb H. IL-1 and TNF independent pathways mediate ICAM-1/VCAM-1 up-regulation in ischemia reperfusion injury. J Leukoc Biol. 2001;70:192–198. [PubMed] [Google Scholar]
- Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of GP130-signaling cytokines and of the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- Daemen MA, vant’t Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol. 1999;162:5506–5510. [PubMed] [Google Scholar]
- Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia reperfusion injury. Cardiovasc Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Friedrichs GS, Homeister JW, Lucchesi BR. The complement system in myocardial ischemia/reperfusion injury. Cardiovasc Res. 1994;28:437–444. doi: 10.1093/cvr/28.4.437. [DOI] [PubMed] [Google Scholar]
- Weiser MR, Gibbs SA, Valeri CR, Shepro D, Hechtman HB. Anti-selectin therapy modifies skeletal muscle ischemia and reperfusion injury. Shock. 1996;5:402–407. doi: 10.1097/00024382-199606000-00003. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Lucchesi BR. Complement activation and inhibition in myocardial ischemia and reperfusion injury. Annu Rev Pharmacol Toxicol. 1994;34:17–40. doi: 10.1146/annurev.pa.34.040194.000313. [DOI] [PubMed] [Google Scholar]
- Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209–222. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet a-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Bevilacqua MP, Moore KL, Mcintyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Clin Invest. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. EMBO J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci USA. 1997;94:757–761. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble JR, Skinner MP, Berndt MC, Vadas MA. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science. 1990;249:414–417. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MB, Ma M, Georg S, Zhou X, Xia J, Finco O, Han S, Kelso G, Howard RG, Rothstein TL, Kremmer E, Rosen FS, Carroll MC. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;25:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos J, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV. Complement and renal ischemia-reperfusion injury. Am J Kidney Dis. 2001;38:430–436. doi: 10.1053/ajkd.2001.26113. [DOI] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA. Carelton’s Histological Technique. Oxford: Oxford University Press,; 1980:pp 228–229. [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- de Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- Kubes P, Kanwar S. Histamine induces leukocyte rolling in post-capillary venules. A P-selectin-mediated event. J Immunol. 1994;152:3570–3577. [PubMed] [Google Scholar]
- Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Lachmann PJ, Hughes-Jones NC. Initiation of complement activation. Springer Semin Immunopathol. 1984;7:143–162. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- Zizzi HC, Zibari GB, Granger DN, Singh I, Cruz LD, Abreo F, McDonald JC, Brown MF. Quantification of P-selectin expression after renal ischemia and reperfusion. J Pediatr Surg. 1997;32:1010–1013. doi: 10.1016/s0022-3468(97)90388-2. [DOI] [PubMed] [Google Scholar]
- Sanders WE, Wilson RW, Ballantyne CM, Beaudet AL. Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood. 1992;80:795–800. [PubMed] [Google Scholar]