Abstract
In this study, the importance of angiogenesis (the growth of new blood vessels from existing ones) for the growth of retinoblastoma was investigated by a retrospective immunohistochemical analysis. An individual vessel index for each tumor was determined using the endothelial-specific antibody CD 31 for vessel staining. The obtained data were correlated with clinical features, pathohistological characteristics, and the presence of metastasis. In 107 retinoblastomas collected between 1980 and 1990, we found no difference in the vessel densities between uni- and bilateral retinoblastomas (P = 0.41). However, tumors that had invaded the chorioid and/or the optic nerve statistically showed higher vessel densities than tumors without local invasive growth (P = 0.05 and P = 0.024). A tendency of higher vessel densities in retinoblastomas presenting with metastasis at the time of diagnosis was observed (P = 0.11). Based on this observation, we proceeded to examine all retinoblastomas presenting with metastasis at the time of diagnosis. These included patients that were treated between 1968 and 1993. The 18 investigated retinoblastomas had significantly higher vessel densities than all other retinoblastomas presenting without metastasis (P = 0.025). Our data indicate that in retinoblastoma, blood vessels are essential for local and systemic invasive growth. Therefore, an anti-angiogenic therapy could be considered in the multimodal therapy concept for retinoblastomas with invasive growth, both locally and systemically.
Retinoblastoma is the most frequent ophthalmic tumor in childhood.1–3 One or both eyes could be affected. Retinoblastomas can harbor the classical mutation of the retinoblastoma tumor suppressor gene.4 Retinoblastomas are thought to develop from immature neuroectodermal cells of the retina.5 Most children become symptomatic with signs of amblyopia or a typical light reflection in the affected eye. Early diagnosis can lead to an excellent outcome with a multimodal therapy concept consisting of chemoreduction, cryo-, laser- or brachytherapy, external beam radiation or enucleation.6 However, patients initially presenting with metastasis have a poor prognosis.7
Retinoblastomas can show endophytic or exophytic growth, sometimes with signs of invasiveness. Tumor cells can invade the choroid or the optic nerve. If the sclera or the lamina cribrosa is invaded, extraocular growth has occurred. By such continual growth, tumor cells can spread to the brain, the surrounding head bones, or soft tissues. In rare cases, retinoblastoma can disseminate in the whole body and form metastasis in the bone marrow, the liver, or the skeletal system. Invasion of the choroid or the optic nerve are risk factors for developing such metastases.8,9
Tumor growth and the formation of hematogenous metastasis depend on angiogenesis.10 The expansion of tumors beyond the size of a few millimeters in diameter, requires the induction of new capillaries. This neovascularization is believed to be mediated by angiogenic stimulators produced and released by stromal cells or tumor cells themselves.11 Numerous studies have demonstrated that the vascular density of a tumor correlates with metastasis and poor outcome.12–15 The importance of angiogenesis for the growth of retinoblastomas has not yet been demonstrated. The expression of angiogenic factors such as VEGF and bFGF have been found, implicating a possible angiogenic potential of retinoblastomas.16,17 In the present study, we have quantified vessels in retinoblastoma tissue with different clinical characteristics and pathohistological features. Our intention in this study was to define the role of angiogenesis in retinoblastomas with regard to invasion and the formation of metastasis. Based on these data one could discuss the possibility of anti-angiogenic therapy in the future.
Materials and Methods
Patients
Between the years 1980 and 1990, 320 eyes were enucleated due to retinoblastoma at our Department of Ophthalmology. Only sections of paraffin-embedded eyes with adequate tumor material to perform immunohistochemical staining were accepted for the study. A total of 107 enucleated eyes with retinoblastomas met this criterion.
For the second part of the study, 30 patients presenting with metastasized retinoblastomas at our Center between 1968 and 1993 were selected. A total of 18 enucleated eyes with retinoblastomas were available for immunohistochemistry.
Immunohistochemistry
Five-micron paraffin sections were stained for tumor vessels with a CD31 antibody (Dako, Copenhagen, Denmark) using an avidin-biotin immunoperoxidase technique, as previously described.18 Hematoxylin and eosin staining was performed in parallel. Hot spots, areas with the richest vascularity, were chosen at low magnification (×40). Then the CD31-positive vessels were counted using an eyepiece with a square field of 0.25 mm2 at a higher magnification (×200). Three hot spots were counted for each tumor independently by two examiners blinded to the clinical and pathohistochemical data. The median of the three counts was recorded. The interobserver counting variability was less than 5%.
Statistical Analysis
Statistical analysis was performed using SPSS 6.01 software (SPSS, Inc., Chicago, IL). The Cox proportional hazards model was applied to select prognostic factors. A P value of less than 0.05 was considered statistically significant.
Results
Initially, a total of 107 retinoblastoma sections were examined. Clinical and pathohistological characteristics are listed in Table 1. Patients presenting with unilateral and bilateral retinoblastomas were included. According to the pathohistological growth pattern, the tumors were evaluated for their invasive growth. The infiltration of the choroid and/or invasion into the optic nerve was defined as local invasive growth. A further criterion was the presence of metastasis at the time of diagnosis.
Table 1.
Pathohistological Characteristics of 107 Retinoblastomas Examined
| Age median | Unilateral bilateral n (%) | No invasion n (%) | Local invasion (choroidea, opticus) n (%) | Extraorbital growth n (%) | Metastasis n (%) |
|---|---|---|---|---|---|
| 19 months | 52 (49%) | 55 (51%) | 52 (49%) | 7 (7%) | 6 (6%) |
| 55 (51%) |
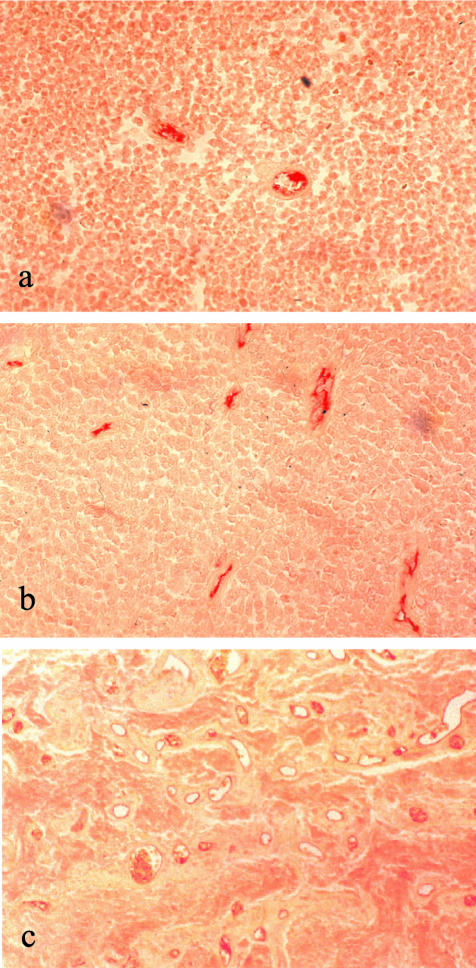
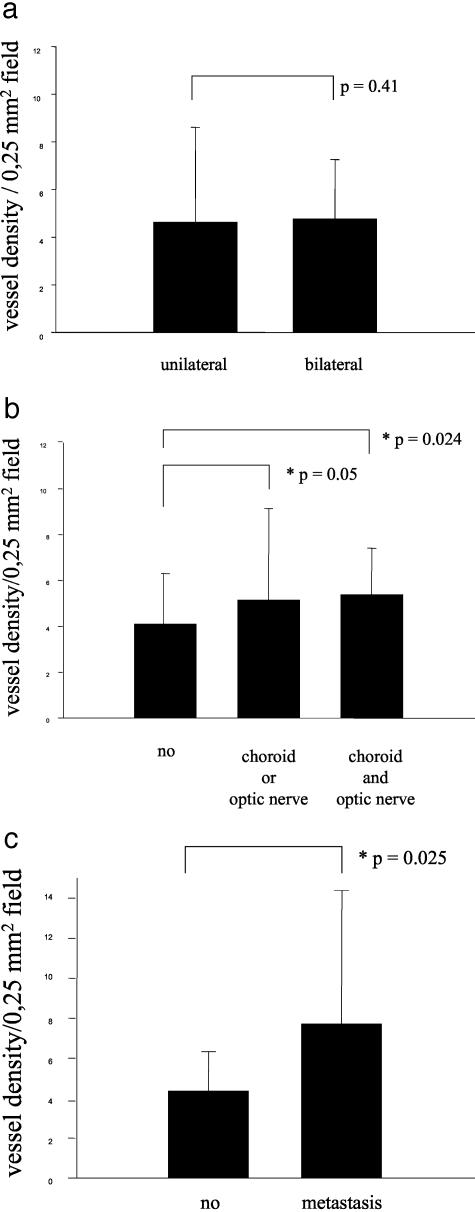
Endothelial cells were stained with a CD31 antibody and vessels were counted according to the international consensus on the methodology and criteria of quantification of angiogenesis in solid tumors.18 A variation of vessel lumen was not observed and the distribution of the vessels in the retinoblastoma showed no particular pattern. Retinoblastomas with scarce and with numerous vessels could be identified (Figure 1, a and b). One retinoblastoma with a very exceptionally high number of vessels could be observed (Figure 1c). Unilateral and bilateral retinoblastomas did not show differences in their median vessel count (unilateral x = 4.6; bilateral x = 4.8; P = 0.41) (Figure 2a). The same was the case with other clinical data, such as age at the time of diagnosis, and sporadic or familial retinoblastoma (data not shown). Local invasiveness ie, retinoblastomas infiltrating the choroid or the optic nerve or both, correlated with higher vessel densities (no local invasiveness, x = 4.1; invasion of choroid or optic nerve, x = 5.1, P = 0.05; invasion of choroid and optic nerve, x = 5.3; P = 0.024) (Figure 2b). When we studied the vascular indices of retinoblastomas presenting with metastasis, we noted a two-fold higher vascular density. This observation was however statistically insignificant (metastasis, x = 10.5; no metastasis, x = 4.7; P = 0.11) (data not shown). The number of patients presenting with metastasis being small (n = 6) possibly plays a role for the insignificant result in the statistical tests. To further investigate the possible correlation between higher vessel densities and the presence of metastasis, we examined all tumors (n = 18) available from retinoblastoma patients presenting with metastasis at the time of diagnosis at our Center between 1968 and 1993. The metastasized retinoblastomas had significantly higher vessel densities than all retinoblastomas presenting without metastasis (metastasis, x = 7.7; P = 0.025) (Figure 2c).
Figure 1.
Immunohistochemical staining for endothelial cells with CD31 antibody in retinoblastoma. a: Poorly vascularized tumor. b: Well vascularized tumor. c: Tumor with very exceptionally high number of vessels. Peroxidase, magnification: ×200.
Figure 2.
Comparison of vessel densities per 0.25-mm2 field. a: Unilateral versus bilateral. b: No local invasive growth versus invasive (choroid or optic nerve involvement, and both choroid and optic nerve involvement). c: No metastasis versus metastasis.
In conclusion, our study demonstrates for the first time that vessel densities in retinoblastoma correlate with local invasive growth and the presence of metastasis at the time of diagnosis. Therefore, in retinoblastoma, angiogenesis seems to play an important role for invasive growth, both locally and systemically.
Discussion
The survival rate of retinoblastoma patients has dramatically improved over the last century. It is now greater than 90% in major centers, while untreated tumors are still mostly fatal.19 Early diagnosis and improved treatment methods are mostly responsible for the improved survival. However, patients from countries with insufficient health care and diagnostic tools still often present with disseminated disease. Such advanced tumors are often not curable with the available multimodal therapy. The survival rate in the cases with presence of metastasis is 30%. The presence of metastasis is, therefore, an important feature in the outcome of retinoblastoma. Risk factors for metastasis in retinoblastomas have been studied by several authors. Pathohistological signs of invasion of eye structures such as the choroid, sclera, and optic nerve anterior or posterior to the lamina cribrosa are highly predictive for the presence of metastasis and correlate with the risk of death.20 In such patients, prophylactic orbital external beam radiotherapy and/or systemic chemotherapy may be indicated. Several studies on patients with metastasis show that high-dose chemotherapy with autologous bone marrow rescue is a therapeutic option, but nevertheless the prognosis is very poor.21,22 Data on biological factors influencing the difference in aggressiveness and invasive potential of retinoblastoma are scarce. The biological phenomenon of tumor angiogenesis as an indication of higher malignant potential has been shown on multiple occasions with solid neoplasms.12–15 In our study, we were interested in whether vessel densities, as an indicator of tumor angiogenesis, play a role in invasive growth of retinoblastomas and the formation of metastasis. If this were the case, anti-angiogenesis could be discussed as a new therapeutic option.
We did not find a difference in vessel densities when clinical data, such as uni- or bilateral retinoblastoma, familial or sporadic cases, sex or age were examined. The first important result of this study shows that the number of intratumoral vessels plays a significant role in local invasive growth. Retinoblastomas are usually detected by fundoscopy at a very early stage when local structures are not invaded by the tumor cells. We speculate that the angiogenic switch,23 which renders the tumor a highly vascularized phenotype, occurs at the stage when the choroid or the optic nerve are infiltrated. In such cases, as shown by our data, angiogenesis seems to play a crucial role for the further expansion. The second important result of our study is that intratumoral vessel densities are a statistically significant factor for the hematogenous metastasizing of retinoblastomas. Once tumor cells have gained access to the circulation, they can colonize to distant sites. Therefore, retinoblastomas with high vessel counts indicate a high risk of developing hematogenous metastasis. As a consequence, in such patients a specific diagnostic and staging program should be initiated to identify metastasis.
Anti-angiogenesis as a new treatment option has been tested for several tumors including pediatric malignancies.24,25 Based on current knowledge, it is believed that patients would profit most from a combination therapy consisting of anti-angiogenic and chemotherapeutic drugs. Such a combination therapy targets both the endothelial compartment and the tumor cell compartment, and seems to be more effective in improving the outcome than either therapy alone.
Retinoblastomas with pathohistological signs of invasiveness are at risk of having metastasized. In regard to our data, tumors additionally demonstrating high vessel densities could profit from an anti-angiogenic treatment. In these rare cases the additional application of anti-angiogenic substances in combination with a chemotherapeutical regime seems to be justified, since we have shown that angiogenesis is an important feature in these tumors.
Footnotes
Address reprint requests to Jochen Rössler, Clinic IV: Pediatric Hematology, Oncology, University Hospital of Freiburg, 79106 Freiburg, Germany. E-mail: roessler@kikli.ukl.uni-freiburg.de.
Supported by the Faculty of Medicine of the University of Essen (IFORES) and Deutsche Leukämie-Forschungshilfe.
Present address for J.R. is Clinic IV, Pediatric Hematology, Oncology, University Hospital of Freiburg, 79106 Freiburg, Germany.
Present address for H.P. and L.S. is Department of Pediatrics 1, Children’s Hospital, University of Göttingen, 37075 Göttingen, Germany.
References
- Kock E, Naeser P. Retinoblastoma in Sweden 1958–1971: a clinical and histopathological study. Acta Ophthalmol Copenh. 1979;57:344–350. doi: 10.1111/j.1755-3768.1979.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Mahoney MC, Burnett WS, Majerovics A, Tanenbaum H. The epidemiology of ophthalmic malignancies in New York State. Ophthalmology. 1990;97:1143–1147. doi: 10.1016/s0161-6420(90)32445-4. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Draper GJ, Kingston JE. Retinoblastoma in Great Britain 1969–80: incidence, treatment, and survival. Br J Ophthalmol. 1988;72:576–583. doi: 10.1136/bjo.72.8.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW. The retinoblastoma tumor suppressor protein. Curr Opin Genet Dev. 1995;5:79–83. doi: 10.1016/s0959-437x(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Tso MOM, Zimmermann LE, Fine BS. The nature of retinoblastoma II: photoreceptor differentiation: an electron microscopy study. Am J Ophthalmol. 1970;69:350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- Bornfeld N, Schüler A, Bechrakis N, Henze G, Havers W. Preliminary results of primary chemotherapy in retinoblastoma. Klin Pädiatr. 1997;209:216–221. doi: 10.1055/s-2008-1043953. [DOI] [PubMed] [Google Scholar]
- Messmer EP, Heinrich T, Hopping W, de Sutter E, Havers W, Sauerwein W. Risk factors for metastasis in patients with retinoblastoma. Ophthalmology. 1991;99:136–141. doi: 10.1016/s0161-6420(91)32325-x. [DOI] [PubMed] [Google Scholar]
- Shields C, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Ophthalmol. 1993;77:544–548. doi: 10.1136/bjo.77.9.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields C. Optic nerve invasion of retinoblastoma: metastatic potential and clinical risk factor. Cancer. 1994;73:692–698. doi: 10.1002/1097-0142(19940201)73:3<692::aid-cncr2820730331>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in medicine of the Beth Israel Hospital, Boston: clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Fox SB, Leek RD, Bliss J, Mansi JL, Gusterson B, Gatter KC, Harris AL. Association of tumor angiogenesis with bone marrow micrometastases in breast cancer patient. J Natl Cancer Inst. 1997;89:1044–1049. doi: 10.1093/jnci/89.14.1044. [DOI] [PubMed] [Google Scholar]
- Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, Muraoka R. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997;57:1043–1046. [PubMed] [Google Scholar]
- Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc amplification, and poor outcome in human neuroblastoma. J Clin Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- Foss AJE, Alexander RA, Jefferies LW, Hungerford JL, Harris AL, Lightman S. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996;56:2900–2903. [PubMed] [Google Scholar]
- Pe’er J, Neufeld M, Baras M, Gnessin H, Itin A, Keshet E. Rubeosis iridis in retinoblastoma: histologic findings and the possible role of vascular endothelial growth factor in its induction. Ophthalmology. 1997;104:1251–1258. doi: 10.1016/s0161-6420(97)30150-x. [DOI] [PubMed] [Google Scholar]
- Schweigerer L, Neufeld G, Gospodarowicz D. Basic fibroblast growthfactor is present in cultured human retinoblastoma cells. Invest Ophthalmol Vis Sci. 1987;28:1838–1843. [PubMed] [Google Scholar]
- Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY. Quantification of angiogenesis in solid human tumors: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;14:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Höpping W, Havers W, Passarge E. Retinoblastom. Bachmann KD, editor. New York: Georg Thieme Verlag, Gustav Fischer Verlag; Pädiatrie in Praxis und Klinik. 1988:pp 755–770. [Google Scholar]
- Olver J, McCartney ACE, Kingston J, Hungerford J. Histological indicators of the prognosis for survival following enucleation for retinoblastoma. Bornfeld N, Gragoudas ES, Höpping W, Lommatzsch PK, editors. New York: Kugler; Tumors of the Eye. 1991:pp 59–67. [Google Scholar]
- Saleh R, Gross S, Cassano W, Gee A. Metastatic retinoblastoma successfully treated with immunomagnetic purged autologous bone marrow transplantation. Cancer. 1988;62:2301–2303. doi: 10.1002/1097-0142(19881201)62:11<2301::aid-cncr2820621107>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sandri A, Besenzon L, Acquaviva A, Marino C, Cordero di Montezemolo L, Madon E. “Eight drugs in one day” chemotherapy in a nonfamilial bilateral retinoblastoma with recurrent cerebrospinal fluid metastasis. Pediatr Hematol Oncol. 1998;15:557–561. doi: 10.3109/08880019809018319. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Schweigerer L. Anti-angiogenesis as a novel therapeutic concept in pediatric oncology. J Mol Med. 1995;73:497–508. doi: 10.1007/BF00198901. [DOI] [PubMed] [Google Scholar]
- Pavlakovic H, Havers W, Schweigerer L. Multiple angiogenesis stimulators in a single malignancy. Angiogenesis. 2001;4:259–262. doi: 10.1023/a:1016045012466. [DOI] [PubMed] [Google Scholar]