Abstract
Injuries to the central nervous system (CNS) trigger an inflammatory reaction with potentially devastating consequences. In this report we compared the characteristics of the inflammatory response on spinal cord injury (SCI) caused by a stab wound between the T7 and T9 vertebrae and spontaneous experimental autoimmune encephalomyelitis (EAE). SCI and EAE were compared in two types of myelin basic protein Ac1-11-specific T-cell receptor transgenic mice: T/R+ mice harbor regulatory T cells, and T/R− mice lack regulatory T cells. Our results show that 8 days after SCI, T/R− mice developed a strong T-cell infiltrate in the spinal cord, with remarkable down-modulation of CD4 expression that was accompanied by a local increase in Mac-3+ and F4/80+ reactivity and diffuse local and distal astrogliosis. In contrast, T/R+ mice exhibited a modest increase in CD4+ cells localized to the site of injury, without CD4 down-modulation; focal astrogliosis was restricted to the site of the lesion, although Mac-3+ and F4/80+ cells were also present. Similarly to T/R− mice that underwent SCI, T cells displaying down-modulated CD4 expression were found in the CNS of older T/R− mice afflicted by spontaneous EAE. Overall, our results suggest that common mechanisms regulate T-cell accumulation in CNS lesions of different causes, such as mechanic lesion or autoimmune-mediated damage.
Cell infiltration and accumulation are features of the tissue response to distinct stimuli, such as traumatic injury, infections, or under some circumstances, self-components. Whatever leads to the presence of leukocytes in tissues, the result ranges from the development of chronic inflammation to a successful resolution. In organs such as the brain, even small lesions can cause important physiological consequences, physical impairment, and behavioral alterations, according to the severity of the response and its localization. Mechanical central nervous system (CNS) lesions can cause primary damage to axons and result in inflammatory reactions1,2 that lead to migration of several types of leukocytes, including T lymphocytes. T lymphocytes are activated when they contact the cognate MHC-peptide complexes. Additional co-stimulatory molecules such as B7 family members participate triggering and modulating the T-cell response.3–5 Most T cells that migrate into the CNS after lesion do not recognize CNS antigens.6 However, in some experimental models it was shown that bystander activation of CD8+ T cells could have a role secondary to the specific Ag recognition, causing tissue damage.7,8
Because CNS-specific T cells are rare, the role of CNS-specific T cells that infiltrate the CNS as a consequence of traumatic injury is not completely understood. To address this question, we took advantage of a mouse model in which a large number of the circulating T cells are specific for myelin basic protein (MBP).9 This experimental system allowed us to address the role of myelin-specific T cells on CNS stab wound lesions and correlate it with development of other CNS pathologies, such as experimental autoimmune encephalomyelitis (EAE).
Mice harboring large number of cells expressing T-cell receptor (TCR) specific for MBP Ac1-11 were previously generated.9 These animals, designated T/R+ mice, have a very high frequency of T lymphocytes that recognize the MBP Ac1-11 peptide presented by the I-Au MHC class II molecule. Despite the fact that the vast majority of CD4+ T cells in T/R+ mice are MBP-specific and immunocompetent, T/R+ animals do not develop any spontaneous CNS pathology. The presence of functional RAG genes in T/R+ mice allows some degree of recombination of endogenous TCR genes, generating a small number of lymphocytes with a diverse recognition repertoire. When T/R+ mice were bred with RAG1-deficient mice, generating T/R− mice, 100% of the animals developed EAE spontaneously.9 Although the number of anti-MBP CD4+ T cells is essentially the same between T/R+ and T/R− mice, a comparison of lymphocytic populations between T/R+ and T/R− mice indicated that T/R− mice lack: 1) a small population of αβ T cells expressing TCR encoded by the endogenous (nontransgenic) α and β loci, including some CD8+ T cells; 2) γδ T cells; and 3) B cells, which are present in normal numbers in T/R+ mice. Because of the RAG mutation, T/R− mice harbor exclusively MBP-specific T lymphocytes. In subsequent studies we crossed T/R+ mice with β2-microglobulin-deficient mice, which lack CD8+ T cells, with μMT mice, which lack mature B cells, with TCRδ gene-deficient mice, which lack γδ-T cells, and with TCRα and TCRβ gene-deficient mice, which can express transgenic MBP-specific TCR chains but not endogenous TCRα and TCRβ chains, respectively.10 Our results, as well as those of others, showed that, insofar as prevention of spontaneous EAE, only CD4+ T cells expressing endogenous αβ TCRs were essential.10,11
Ensuing studies determined that not all αβ CD4+ T cells are protective against EAE, ruling out competition models, and eventually supporting a key role for the specificity of the TCRs expressed by regulatory T cells.12 However, spontaneous EAE in T/R− mice could be suppressed by the presence of regulatory CD4+ T cells. For the regulatory function, the expression of the CD25 marker, which is traditionally associated with regulatory T cells,13 is not necessary, although an interleukin (IL)-2-dependent in vivo conversion of CD25− regulatory T cells into CD4+CD25+ T cells correlates with the acquisition of regulatory functions and Foxp3 expression.14,15 We and others also transferred total spleen cells or purified CD4+ cells from wild-type syngeneic animals and showed that they could confer protection against the development of spontaneous EAE when administered before the onset of disease.10,11 Therefore, a regulatory pool of cells is present in normal individuals, which is contained within the population of αβ CD4+ cells.
The role of the regulatory mechanisms on CNS lesions is an extremely relevant point in addition to the role of CNS-specific effector T cells, leading to insights into the evolution and repair of CNS lesions. To further understand the regulation of CNS lesions, T/R+ and T/R− mice were subjected to a stab wound made between the T7 and T9 vertebrae in the spinal cord. The frequency of antigen (Ag)-specific cells and their activation status was determined in animals of different ages. The phenotype of the infiltrating lymphocytes was compared to spontaneous EAE. We found significant differences in the extent of the inflammatory response in animals that harbor (T/R+) or do not harbor (T/R−) regulatory T cells. These differences parallel those found in spontaneous EAE. We conclude that T-cell responses, both harmful and protective, follow common pathways regardless of the triggering signal being a mechanical lesion or a spontaneously developing autoimmune disease.
Materials and Methods
Mice
MBP Ac1-11-specific transgenic TCR mice (T/R+ and T/R−) on B10.PL genetic background (H-2u) were previously described.9 To generate T/R− mice, T/R+ mice were crossed to RAG-1 knockout mice.16 All animals were maintained under SPF conditions at the Skirball Institute Animal Facility, New York University Medical Center.
EAE Score
EAE was scored as described by Baron and colleagues17: level 1, limp tail; level 2, weak or partial leg paralysis; level 3, total hind leg paralysis; level 4, hind leg paralysis and weak or partial front leg paralysis; level 5, moribund (immediately sacrificed). Protocols were approved by New York University Institutional Animal Care and Use Committee.
Stab Wound Injury in the Spinal Cord (SCI)
The mice were anesthetized with an intraperitoneal injection of 0.2 ml of a 2.5% solution of 2,2,2-tri-bromo-ethanol (Sigma, St. Louis, MO) in distilled water. The level of anesthesia was evaluated by tests of cornea reflex to peripheral pain stimulus. The surgical area was shaved and disinfected with iodide. The animals were placed on a heated surface, for a cutaneous incision on the dorsal area between T1 and T12. The muscle on the T8 region was exposed to allow the visualization of the vertebrae under a scope. The localization of T8 was made based on the fact that T13 corresponds to the vertebrae associated to the last rib, with retrograde count. A laminectomy was performed and a 22½-gauge needle was inserted for 2 mm at a 45-degree angle. After suture, the animals were maintained under heat. All procedures were approved by the New York University Institutional Animal Care and Use Committee.
Spinal Cord Dissection and Single-Cell Suspension
Mice were anesthetized with Metofane (Metoxifluorane; Mallinckrodt Veterinary, Inc., Mundelein, IL), to obtain a 50-μl blood aliquot from orbital plexus. After a 10-minute perfusion with phosphate-buffered saline (PBS) containing 5 mmol/L ethylenediamine tetraacetic acid, the vertebral column was removed and divided in four segments with equivalent length: R1 (from the cervical C1 to the thoracic T2), R2 (from T3 to T9), R3 (from T10 to the lumbar L4), and R4 (from L5 to Co3). Dissected segments were placed in separate wells of a 48-well plate. Blood cells were submitted to the same treatment for control. To each well, 100 μl of a 10-mg/ml type VI collagenase was added. The samples were incubated for 1 hour at 37°C, additionally disrupted with a pipette, and passed through a 45-μm nylon mesh. The volume of each sample was completed to 860 μl to which 530 μl of an 89% Percoll-saline solution was added. The samples were centrifuged at 300 × g for 30 minutes. The pellet was resuspended in 100 μl of PBS containing 5% fetal calf serum and 0.1% sodium azide (staining buffer), washed twice in the same buffer, and incubated 45 minutes with the respective labeled antibodies for flow cytometric analysis of phenotype (2.5 μg/106 cells). After this period, the cells were washed twice and resuspended in propidium iodide for exclusion of dead cells and debris.
Flow Cytometry
Cells isolated as described (2 × 105 to 10 × 105) were stained with 50-μl mixtures of antibodies diluted according to a previous titration in staining buffer (Hanks’ balanced salt solution with 2% fetal calf serum and 0.01% NaN3). The antibodies used for the staining were anti-CD3 (Pharmingen, San Diego, CA), anti-CD4 (Pharmingen), and an anti-MBP TCR clonotypic monoclonal antibody, 3H12. 3H12 is a mouse IgM generated in our laboratory as described;10 it recognizes the transgenic MBP-specific T cells while staining virtually no cells in B10.PL nontransgenic littermates.10 Isotype controls (Pharmingen) were also used. The cells were then processed through a FACScalibur flow cytometer before analysis of data with CellQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, CA).
Histopathology and Immunohistochemistry
Tissues were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm sections. Sections were serially assembled on slides, labeled according to animal numbers and without reference to experimental groups, to avoid biased analysis. After hematoxylin and eosin (H&E) staining, sections from each tissue block were examined microscopically. Serial sections were immunohistochemically stained. Briefly, 5-mμ sections mounted on charged slides were deparaffinized and hydrated. Endogenous peroxidase was blocked by a 30-minute treatment with 0.3% H2O2 in 100% P.A. methanol. Nonspecific binding was blocked with 5 g/L casein in PBS solution, pH 7.4, in a PBS-humidified chamber. The slides were incubated overnight with the primary antibody anti-GFAP (DAKO, Carpinteria, CA), or anti-Mac 3 (Pharmingen), or anti-F4/80 (clone X2; kindly donated by Dr. Nora Sarvetnick, the Scripps Research Institute, La Jolla, CA), diluted to 1.5 μg/ml in casein block solution, under low agitation, at 4°C. After that, slides were incubated for 1 hour with biotin-labeled anti-rat IgG (Vector Laboratories, Burlingame, CA), followed by a 30-minute incubation with streptavidin-peroxidase (Zymed Laboratories Inc., San Francisco, CA). Color was developed with NovaRed (Vector Laboratories), according to the manufacturer’s instructions. Slides were counterstained with Gill’s hematoxylin, rehydrated, mounted, and microscopically evaluated.
Magnetic Cell Sorting
Splenic cells were incubated with bead-labeled anti-CD4 antibodies (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s protocol, and passed through VarioMacs magnetic columns (Miltenyi Biotec) attached to a magnet. The purity of CD4-enriched and -depleted fractions was checked by fluorescence-activated cell sorting (FACS), before in vivo injection.
Cell Transfer
One-week-old T/R− mice received intraperitoneally 2 × 107 total splenic cells, magnetically sorted CD4+ cells, or unbound, CD4-depleted fraction, from wild-type mice. At 4 to 5 weeks of age, circulating CD4+ 3H12− cells were quantified by FACS. At 4 or 5 weeks of age, SCI was performed as described.
Statistical Analysis
Normality and homogeneity of variances were verified by Tukey and Bartlett’s test, respectively. Samples were compared using Kruskal-Wallis nonparametric analysis of variance, followed by Dunn’s multiple comparisons. Statistical analysis was performed using GraphPad Prism Software (San Diego, CA).
Results
A stab wound spinal cord injury (SCI) was performed on the T7 to T9 vertebral segment in the spinal cord of T/R+ and T/R− animals. Both T/R+ and T/R− mice harbor a large number of MBP-specific T cells, but T/R+ mice also have a small population of T cells displaying capacity to prevent the occurrence of spontaneous EAE. In contrast, the T-cell repertoire in T/R− mice consists exclusively of MBP-specific T cells.9–11,13
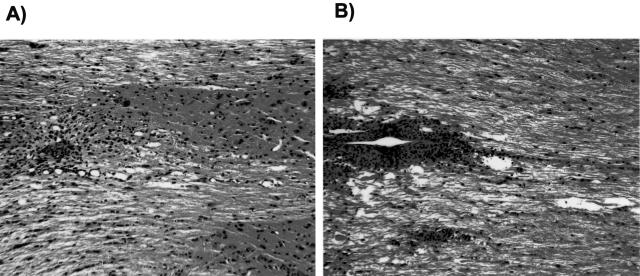
We compared the histopathology of T/R+ and T/R− mice in H&E-stained sections from the site of the lesion, and from the lumbar region, which is distant from the stab wound. Analysis of the site of the lesion showed remarkable differences between the two groups. Figure 1 shows representative 5-week-old animals from the T/R+ (A) and T/R− (B) groups, 8 days after a SCI. At this time point it was possible to distinguish between the two groups based on the density and distribution pattern of infiltrating cells. T/R− mice showed a much higher number of infiltrating cells with a focal as well as diffuse pattern of distribution, with a large area of lesion and edema, as opposed to T/R+ mice, which had a significantly lower density, smaller and at most focally restricted infiltrate. Overall, at 8 days after SCI, the T/R− mice developed a lesion with a larger number of cells and denser pattern of infiltration than did the T/R+ animals. Two additional types of analysis provided support for the conclusions reached on examination of the H&E-stained specimens: flow cytometry and immunohistochemistry.
Figure 1.
Histological analysis of the spinal cord from 5-week-old T/R− and T/R+ mice. Eight days after stab wound lesion (SCI) at the T7 to T9 vertebrae level, samples were prepared for T/R+ (A) and T/R− mice (B). The sections are representative of five animals per each group, and were stained with H&E. Original magnifications, ×16.
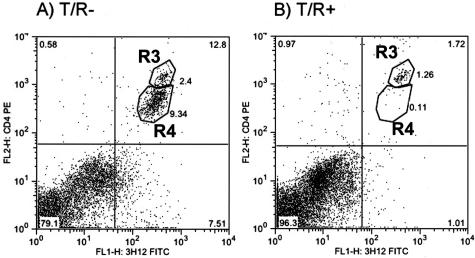
The infiltrating cells in both groups of transgenic animals as well as wild-type controls were characterized by flow cytometry, performed on cell suspensions from segments of the spinal cord. The initial characterization of cell populations was made using anti-CD4 and the anti-clonotypic antibody 3H12, which recognizes the MBP-specific TCR.10 Figure 2 shows the percentage of CD4+ 3H12+ cells in the T7 to T9 segment of the spinal cord of representative 5-week-old animals from T/R− (A) and T/R+ (B) groups.
Figure 2.
Down-modulation of CD4 and TCR in MBP-specific (3H12+) cells from T/R− mice subjected to SCI. Eight days after SCI, spinal cord samples from 5-week-old T/R− (A) and T/R+ mice (B) were prepared for FACS analysis. Note the appearance of CD4-low cells (contained within the region labeled R4) in T/R− but not T/R+ mice. Representative of 13 animals per group.
Because all of the events from the T7 to T9 region were collected in the cytometer, it was possible to compare absolute and relative numbers of MBP-specific T lymphocytes, based on the number of events. Eight days after SCI of 5-week-old mice, there were 4600 ± 2100 MBP-specific T cells in the T7 to T9 region of T/R− mice, compared to only 296 ± 95 cells in T/R+ mice. Because there are exceedingly few 3H12+ cells in wild-type mice, in these animals we determined the infiltration by total CD4+ cells. This number was 25 ± 16 cells (n = 17, 7, and 4, for T/R−, T/R+, and wild-type mice, respectively). We also performed the SCI procedure in animals of different ages (4, 5, and 6 weeks old), and performed flow cytometry analysis of the MBP-specific T-cell infiltrate. These data are presented in Table 1.
Table 1.
Percentage of MBP-Specific CD4+3H12+ T Cells in the T7–T9 and Segment of the Spinal Cord in T/R+ and T/R− Mice at Different Ages, 8 Days after SCI, or after Mock Surgery
| Age | T7–T9
|
Lumbar
|
||||
|---|---|---|---|---|---|---|
| NT/R+* | T/R+† | T/R−† | NT/R+* | T/R+ | T/R− | |
| Mock | ||||||
| 4 weeks | 0.0 ± 0.0 | 0.02 ± 0.01 | 0.05 ± 0.03 | 0.01 ± 0.0 | 0.05 ± 0.05 | 0.065 ± 0.02 |
| 5 weeks | 0.0 ± 0.0 | 0.03 ± 0.02 | 0.18 ± 0.08 | 0.01 ± 0.01 | 0.11 ± 0.06 | 0.12 ± 0.05 |
| 6 weeks | 0.01 ± 0.01 | 0.07 ± 0.04 | 0.03 ± 0.01 | 0.0 ± 0.0 | 0.06 ± 0.06 | 0.11 ± 0.05 |
| SCI 8 days | ||||||
| 4 weeks | 1.96 ± 0.4 | 0.17 ± 0.02 | 1.84 ± 0.04 | 0.43 ± 0.1 | 0.08 ± 0.07 | 0.08 ± 0.02 |
| 5 weeks | 1.24 ± 0.2 | 2.15 ± 1.22 | 17.64 ± 3.76† | 0.25 ± 0.15 | 0.44 ± 0.23 | 4.08 ± 1.23† |
| 6 weeks | 1.35 ± 0.3 | 3.16 ± 1.55† | 21.39 ± 8.65† | 0.30 ± 0.12 | 0.52 ± 0.2 | 6.13 ± 2.2† |
The percentage of total CD4+ T cells in wild-type (NT/R+) animals, subjected to SCI or mock surgery, is also shown. The values are the average of six animals per group ± SD.
For wild-type animals (NT/R+), the values correspond to the total CD4+ population. We do not show the frequency of CD4+ 3H12+ cells in wild-type mice because it is below the limit of sensitivity of the method, ie, there is no difference between 3H12-stained and unstained samples.
P < 0.05 between different groups at matching ages.
Table 1 and Figure 2 illustrate several important points. First, consistent with the histological analysis, the number of infiltrating T cells is significantly higher in T/R− mice than in T/R+ mice or wild-type mice subjected to SCI. The clear differences in the number (above) and frequency of infiltrating cells shown in Figure 2 8 days after SCI were, however, not apparent 48 hours after lesion (data not shown). Animals subjected to mock surgery did not show any appreciable inflammatory infiltrate (Table 1).
Second, age-dependent differences in the accumulation of infiltrating lymphocytes were observed on SCI in T/R− mice. The T-cell infiltrate that followed the stab wound was, clearly, more intense in 5- and 6-week-old animals than in 4-week-old animals. This is particularly interesting given the fact that clinical manifestations of spontaneous EAE in T/R− mice do not occur before 6 weeks of age, despite the fact that large numbers of immune competent MBP-specific T cells circulate in T/R− mice since birth. Thus, massive accumulation of MBP-specific T cells in the CNS does not take place before 5 weeks of age even when a stab wound is produced.
Third, although the spinal cords of T/R+ mice had much fewer infiltrating T cells than T/R− mice, after 5 weeks of age they harbored much more infiltrating T cells than wild-type B10.PL (NT/R+) mice (above, and Table 1). This is consistent with a previous report by Jones and co-workers18 comparing the same T/R+ mouse strain with wild-type mice.
Fourth, in T/R+ mice, a relatively large proportion of 3H12-negative CD4+ T cells could be found in the spinal cord (Figure 2B, top left). 3H12-negative T cells in T/R+ mice express an array of endogenous TCR chains, mostly TCR α chains;10 expression of endogenous TCR chains confers broad reactivity to the T cells that express them. As we showed previously,9,10 the percentage of 3H12− CD4+ T cells does not exceed 5% of the total CD4+ cells in the periphery of T/R+ mice, but reaches values close to 30% in their injured spinal cord. Thus, it is likely that T cells with non-CNS specificity can be found among infiltrating T cells, in accordance with previously described models.6
Finally, a remarkable down-modulation of the CD4 molecule, and some decrease on TCR expression, were observed on 3H12+ cells in T/R− animals that underwent the SCI procedure. As shown in Figure 2A, based on fluorescent intensity, CD4+3H12+ T cells in these animals can be divided in two well-defined regions, indicated as R3 and R4. In contrast, T cells from similarly treated T/R+ mice only fell into the high CD4, high 3H12-expressing region (indicated as R3, Figure 2B). The down-modulation of CD4 in T/R− spinal cord closely correlated with the ability of MBP-specific T cells to accumulate in the CNS, which was also observed in the spinal cord of animals afflicted with spontaneous EAE (see below).
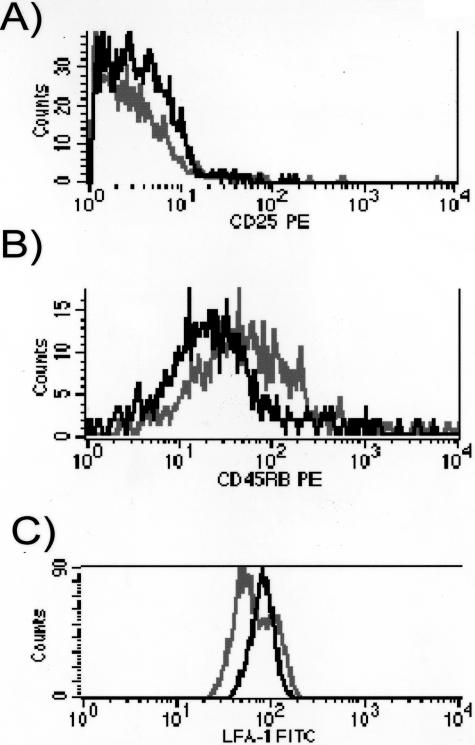
Comparison of the expression of activation markers on the down-modulated (region R4) versus the non-down-modulated CD4+3H12+ population (region R3) in the same spinal cord showed that cells gated on the low-receptor density (black color histograms) had an increased expression of CD25 and CD11a (the α chain of LFA-1), and a lower expression of CD45RB, indicating a higher state of activation, when compared with the nondown-modulated cells (gray histograms) (Figure 3). As expected, CD4+3H12+ T cells in T/R+ mice look like the non-down-modulated cells (R3) in the T/R− spinal cord (not shown).
Figure 3.
Activation status of spinal cord T cells after SCI. Histograms show CD25 (A), CD45RB (B), and CD11a (C) expression on CD4-hi (black line) and low (gray line) cells from T/R− mice. CD4-hi cells correspond to the R3 gate in Figure 2A, and CD4-lo cells correspond to the R4 gate in Figure 2A. These data correspond to 2 animals representative of the analysis of 13 animals per group.
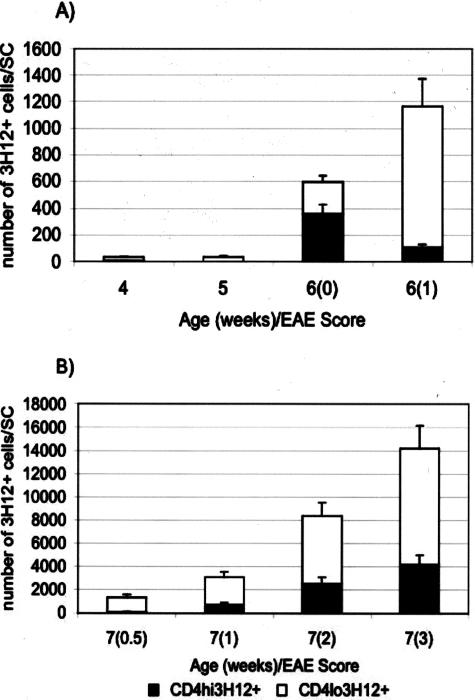
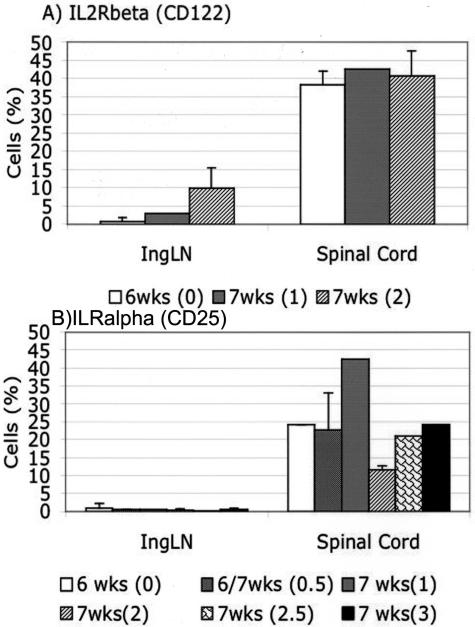
Similarly to the CD4+ T cells that accumulated in the spinal cord of T/R− animals after SCI, cells displaying down-modulated levels of CD4 and TCR were observed in the spinal cord of mice afflicted with spontaneous EAE (Figure 4). Kinetic studies in sham-operated T/R− mice showed that the appearance of a 3H12+ population expressing low levels of CD4 and TCR in the CNS was the best correlate with the occurrence of clinical signs of disease. Disease-free animals at 4, 5, or 6 weeks of age have much fewer T cells in the CNS, and the ones that are there are mostly high-CD4-expressing T cells. This is particularly clear at 6 weeks of age. Once clinical disease is apparent, large numbers of T cells with down-modulated CD4 can be purified from the CNS (Figure 4). With regards to activation markers, the down-modulated T cells prepared from the CNS of spontaneous EAE-afflicted animals are similar to the ones we found in animals that underwent SCI (Figure 5). We have previously shown that T cells from the CNS of EAE-afflicted T/R− mice expressed higher CD11a and lower CD45RB levels.9 Figure 5 shows that, similarly to the T cells recovered from the T7 to T9 region after SCI, the T cells recovered from spontaneous EAE displayed an activated phenotype as determined by expression of CD25 and CD122 (respectively the α and β chains of the high-affinity IL-2 receptor complex). Lymph nodes were used as control sites.
Figure 4.
Number of MBP-specific (3H12+) T cells, expressing high and low levels of CD4, in the spinal cord of T/R− animals undergoing spontaneous EAE. T cells were extracted from spinal cords from sham-operated T/R− mice of different ages, before or after EAE onset. The EAE score is indicated in parentheses. A: T/R− animals from 4 weeks to 6 weeks of age, with or without EAE. B: T/R− animals 7 weeks old afflicted with spontaneous EAE of increasing levels. Values correspond to the average ± STD of 9 to 39 mice per group.
Figure 5.
Expression of high- and low-affinity IL2R (CD25 and CD122) on CD4+ 3H12+ cells from inguinal lymph nodes and from the spinal cord of T/R− animals at different ages and scores of disease. Values correspond to the average ± STD of four to seven mice per group.
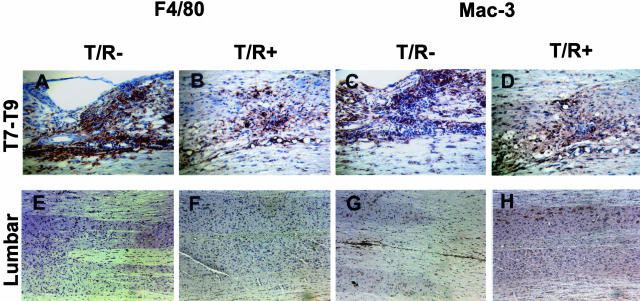
To complement the H&E and flow cytometry analyses described above, we performed immunohistochemical characterization to assess the frequency and activation status of macrophages, microglial cells, and astroglial cells in T/R− and T/R+ mice subjected to SCI. Macrophages and microglia cells were stained for the identification of Mac-3 (a lysosomal antigen equivalent to LAMP-2/CD107b also present on the surface of mature mononuclear phagocytes), and F4/80 (a 160-kd glycoprotein expressed by murine macrophages). F4/80 expression is stronger in activated than in resting microglial cells.19 We performed this analysis in the T7 to T9 injury site, as well as a location in the lumbar spinal cord that is distal to the wound. At the site of injury we could observe that a higher number of F4/80 and Mac-3-positive cells was present in T/R− than in T/R+ specimens (Figure 6, A and B for F4/80, and C and D for Mac-3 expression), indicating that, besides T cells, the effects of injury are extended inflammatory cells. In contrast, no prominent Mac-3 or F4/80 staining was observed in either T/R− or T/R+ samples from the distal locations (Figure 6, E and F for F4/80, and G and H for Mac-3).
Figure 6.
Inflammatory cells in T/R+ and T/R− spinal cords. SCI site (T7 to T9) and lumbar region sections were immunostained for detection of Mac-3 (C, D, G, and H) and F4/80 (A, B, E, and F), 8 days after SCI in T/R+ (B, F, D, and H) and T/R− (A, E, C, and G). A–D: The injury site. E–H: Lumbar area, distant from the SCI site. All slides were stained and color was developed in parallel. The injury site of SCI-subjected T/R− mice displays enhanced Mac-3 and F4/80 reactivity compared to T/R+ mice. In the lumbar region, the presence of F4/80 and Mac-3+ cells was comparable and low. Sections are representative of four animals per group. Original magnifications: ×16 (A–D); ×10 (E–H).
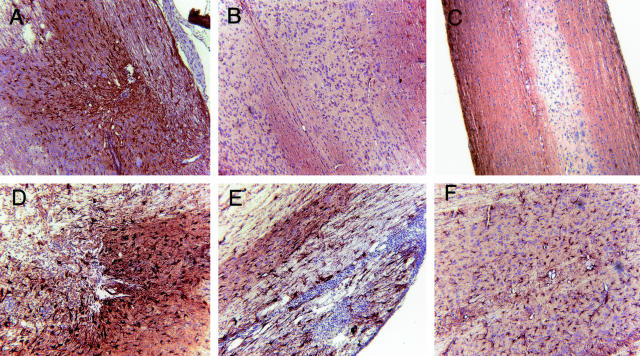
T/R+ and T/R− mice subjected to SCI also differed on astrocyte activation and gliosis. GFAP staining showed that astrocyte activation was greater in the site of lesion in T/R− animals comparatively to T/R+. Furthermore, the area of gliosis was larger, as well as the intensity of GFAP expression which was much stronger in T/R− animals (Figure 7). GFAP+ cells in T/R+ mice were smaller and weakly stained (Figure 7A) in comparison to the same cells in T/R− mice (Figure 7, D and E). We also observed the generation of strong gliosis in the lumbar region of T/R− animals, an area distant from the injury site (Figure 7F). In contrast, T/R+ mice had a very low or no GFAP expression in the lumbar segment of the injured spinal cord (Figure 7B). The gliosis that we observed distant from the injury site in T/R− mice represents a far-reaching reaction to SCI, and not a consequence of the impending spontaneous EAE in these mice, as Mac-3 and F4/80+ cells were in low numbers in that site (Figure 6, E and G). Besides, T/R− controls that underwent mock injury in parallel displayed a significantly milder GFAP staining in the whole extension of the spinal cord (Figure 7C).
Figure 7.
Gliosis in the spinal cord of T/R+ and T/R− mice. Eight days after SCI, GFAP staining was performed in spinal cord samples of T/R+ (A and B) and T/R− mice (C–F). Section from the site of lesion (A, D, and E) can be observed, as well as sections from the lumbar region (B and F) of the spinal cord. GFAP expression was more abundant and stronger on both injury site and distal areas in T/R− mice than in T/R+. C: The lumbar region of an age-matched T/R− mouse subjected to a mock injury procedure. Note the absence of astrogliosis in this sample. Sections are representative of four animals per group. Original magnifications, ×16.
We and others have previously reported that spontaneous EAE in T/R− mice could be prevented by the transfer of spleen cells from wild-type mice before disease onset.10,11,13 We sought to address whether similar protective mechanisms can operate in SCI as well as in spontaneous EAE. One-week-old T/R− mice were reconstituted with cells obtained from spleens of syngeneic wild-type mice (NT/R+). At the age of 4 weeks we assessed the donor cell repopulation of the recipient mice by FACS analysis of peripheral blood. The degree of reconstitution was determined based on the presence of CD4+3H12− cells, which are exclusively of donor origin. Reconstitution levels were determined before the performance of SCI. Three to four percent of CD4+3H12− cells present in the blood were considered as positive reconstitution. Eight days after the lesion, the T7 to T9 segment was dissected, a cell suspension was made, and FACS analysis was used to determine the proportions of infiltrating T cells and their phenotype. Figure 8 shows that the accumulation of transgenic (3H12+) CD4+ T cells was significantly reduced by the reconstitution of T/R− animals with nontransgenic spleen cells. Another important observation was that the MBP-specific T cells in reconstituted T/R− mice did not display down-modulation of CD4 or the TCR. Therefore, the transference of normal splenic cells is beneficial in reducing the consequences of SCI in T/R− mice as in preventing spontaneous EAE. In addition, T/R+ mice, which do not develop spontaneous EAE, have milder lesions on SCI (Figure 1). Altogether, these data suggest similarities in the mechanism of regulation of the inflammatory response in spontaneous EAE and SCI.
Figure 8.
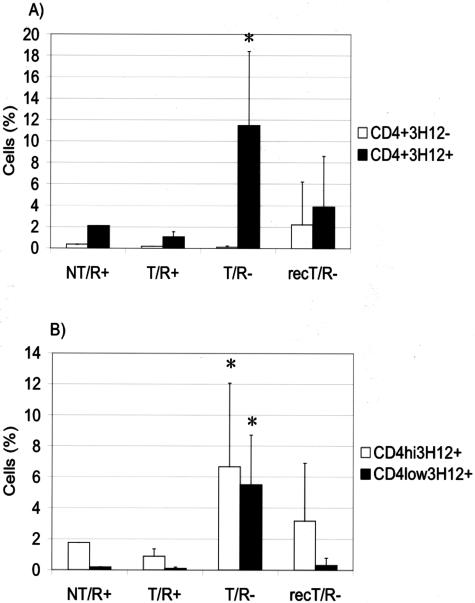
Effect of reconstitution of T/R− mice with wild-type splenocytes on the accumulation of CD4+ cells in the T7 to T9 segment of the spinal cord. T/R− mice were reconstituted with wild-type syngeneic splenocytes. After 4 to 5 weeks, they were subjected to SCI. The spinal cord T-cell populations of splenocyte-reconstituted T/R− mice (recT/R−) were compared to wild-type mice (NT/R+), T/R+ mice, and nonreconstituted T/R− mice, all groups 8 days after SCI. A: Percentage of 3H12+ and 3H12− CD4+ T cells. B: Percentage of 3H12+CD4+ T cells with high and low levels of CD4 expression. Values represent the average of five animals per group. *P < 0.05.
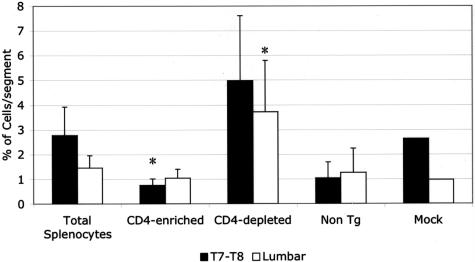
We have previously shown that, in contrast to T/R− mice, spontaneous EAE does not occur in T/R+ mice because of the presence of a small proportion of CD4+ T cells that express endogenous TCR chains (≤5% of total T cells). To clarify whether that CD4+3H12− T-cell population also provides protection in conditions of mechanical lesion, as they do in preventing spontaneous EAE, T/R− animals were transferred with purified splenic CD4 cells, or CD4-depleted splenic cells, and subjected to SCI. As shown in Figure 9, the transfer of unfractionated splenocytes led to the reduction of infiltrating 3H12+ cells into the T7 to T9 region as well as into the lumbar region of injured T/R− mice. The transfer of purified CD4+ cells led to a dramatic reduction of host-derived 3H12+ T cells into the spinal cord, while the CD4-depleted fraction did not display protective activity. Neither non-Tg (wild-type) animals, nor T/R− mice submitted to mock surgery, accumulated significant numbers of CD4 cells in the spinal cord (Figure 9). Thus, the same cells that prevent spontaneous EAE can also reduce the extent of cell accumulation induced by SCI.
Figure 9.
Total splenocytes and purified nontransgenic CD4+ T cells, but not CD4-depleted splenocytes, reduce MBP-specific T-cell load in the spinal cord of T/R− mice subjected to SCI. Neither wild-type animals (non Tg), nor T/R− mice submitted to mock surgery accumulated substantial numbers of CD4 cells in the spinal cord. Data represent the average ± SE of five animals for each group of recipients. *P < 0.05 in comparison to total splenocytes.
Discussion
In this study we compared the dynamics of the inflammatory response in a mouse model of spontaneous autoimmune encephalitis (EAE) and a model of mechanical lesion in the spinal cord (SCI). Spontaneous EAE is developed in T/R− mice but not in T/R+ mice, because of the presence of a regulatory pool of cells in the latter strain. In T/R− animals 5 weeks old or older subjected to SCI, severe tissue damage was noted, whereas equally treated age-matched T/R+ mice displayed much milder lesions. The differences between T/R− and T/R+ are remarkable considering that both T/R− and T/R+ mice have a T-cell repertoire highly dominated by immunocompetent MBP-specific T cells.9,10,13
In perfect correlation with the control of spontaneous EAE,10,11,13 we could also reduce the extent of SCI-induced inflammation in T/R− mice by transfer of polyclonal CD4+ T cells, which contain regulatory properties, but not CD4+ T cell-depleted splenocytes. Our results support a strong parallel between the inflammatory response to SCI and its regulation, and the development and regulation of spontaneous EAE.
The experimental model used in this work allowed us the analysis of the role of myelin-specific T cells in lesion development or aggravation. The infiltration of activated T cells into the CNS has been suggested as one of the main causes of tissue damage leading to motor/cognitive and organic impairment in several experimental models that mimic human diseases.20 Interestingly, in 75% of all mice we observed some level of hind leg paralysis after SCI, which was recovered in T/R+ mice within 48 hours, but not in T/R− mice. One possible explanation for this rapid paralysis is the edema caused by SCI. However, we do not know what causes 25% of T/R− mice to remain free of clinical signs in the immediate aftermath of SCI because edema was observed in all histological preparations from this group. Nevertheless, whatever mechanism is responsible for this phenomena, it is refrained in the presence of a regulatory population. It is possible that the regulation of T-cell infiltrate and inflammation is accompanied by more efficient tissue repair mechanisms, either through the modulation of cell numbers or by changing the quality of the local cell secretion products.
Several clinical and experimental models provide considerable indications that trauma can initiate an autoimmune response, although it may not always be clinically harmful. For instance, sympathetic ophthalmia, a disease in which injury to one eye results in antigen release, immune priming, and autoimmune attack against the other eye that initially was not injured, has been treated with immune suppressants and anti-inflammatory drugs for several decades.21,22 In a model of unilateral facial nerve transection, which is a peripheral nervous system lesion, CD4+ T cells can be observed infiltrating not only the injury site, but also the ipsilateral facial nuclei, with widespread perivascular infiltrates. This effect was more prominent in EAE-susceptible Lewis rats than in the more resistant BN rats.23 Finally, T cells obtained from SCI sites and briefly cultured in vitro with MBP were able to induce EAE in naïve rats. Interestingly, EAE induction was only possible with T cells prepared 7 days after SCI, and did not occur when the same treatment was applied to T cells from noninjured animals.24
The role of trauma in the etiology of multiple sclerosis is not clear, with studies supporting either the point of view that trauma is important or the opposite.25 Many studies published since the 19th century recounted that, in a fraction of promptly diagnosed MS cases (∼10%), there had been anteceding CNS trauma. However, it is clear that CNS trauma, by itself, does not cause MS. It is therefore possible that the inflammatory response caused by trauma triggers reactivity in individuals who are already highly susceptible to the disease due to underlying immunological imbalances. In our experimental model, T/R− mice subjected to injury develop a more severe inflammatory response T/R+ mice, suggesting a role for regulatory populations in providing such a balance.
We observed a highly reproducible down-modulation of CD4 in MBP-specific T cells in the spinal cord of T/R− mice undergoing spontaneous EAE or subjected to SCI. Down-modulation of CD4 in response to antigen exposure was previously noticed in correlation with T-cell activation.26,27 Consistently, CD4-down-modulated MBP-specific T cells in T/R− mice displayed higher levels of activation markers than the CD4-hi T cells extracted from the same mice. However, in most experimental systems, CD4 down-modulation in vivo is not as common and as clear-cut as what we observed here. It has been shown that exposure to gangliosides leads to CD4 down-modulation in vitro;28 as gangliosides are very abundant in the CNS parenchyma, it may contribute to the CD4 down-modulation to be so prominent in our experimental system. However, CNS-infiltrating cells in T/R+ mice did present MBP-specific T cells with down-modulated CD4, suggesting that ganglioside exposure is not responsible for the CD4 down-modulation in this system. We have thus a strong correlation between pathogenic infiltration of CNS parenchyma by MBP-specific T cells and CD4 down-modulation on the same cells. It is possible that the small number of CD4-hi MBP-specific T cells present in T/R+ mice did not become fully activated within the CNS. Thus, the presence of CD4 down-modulation on T cells appears to be a good indicator of the presence of active inflammatory lesions within the CNS.
Co-receptors such as CD4 contribute to the avidity of the interaction between T cells and antigen-presenting cell.29 Thus, co-receptor down-modulation is one of the ways of reducing the avidity of T cell/APC interaction and reduce immune responsiveness. Co-receptor down-modulation on T cells can be achieved experimentally through high levels of antigen expression in peripheral tissues. Using a double-transgenic system in which Kb-specific TCR transgenic mice were crossed to mice expressing Kb under the GFAP promoter, Schonrich and colleagues30 showed that TCR and CD8 co-receptor down-modulation represented a mechanism by which peripheral immune tolerance toward Kb could be achieved. In view of these precedents, it is likely that the CD4 down-modulation that we observe is a reaction that reduces the reactivity of the MBP-specific T cells. Indeed, we observed that T cells from MBP TCR transgenic mice crossed with CD4-null mice are poorly selected in vivo and respond weakly to MBP in vitro (data not shown). However, in the present model CD4 down-modulation is a relatively late event, which is not observed before MBP-specific T cells infiltrate the CNS, and it does not seem to prevent the onset of EAE.
The presence of cells expressing F4/80 and Mac-3, which are characteristic of inflammatory macrophages and activated microglia was also enhanced in T/R− mice subjected to SCI. This reveals that the increased T-cell numbers are accompanied by the infiltration of other leukocytes, particularly with inflammatory properties. Interestingly, although in T/R+ less F4/80 and Mac-3 cells were observed, they were still significantly represented. This suggests that the regulatory process may modulate inflammatory response into beneficial for tissue repair. In some models, the depletion of blood-derived macrophages by the administration of clodonate liposomes reduces the number of macrophages and improves recovery from moderate CNS lesion.31 However, a role for macrophages has been described in oligodendrocyte remyelination.32 In fact, the presence of IL-1β and IGF-1 in the system has beneficial effects over CNS tissue repair and remyelination.33 Further studies on macrophage/microglia-derived factors in T/R+ and T/R− mice subjected to SCI are currently ongoing. Interestingly, in contrast to MBP-specific cells, which were detected in the lumbar portion of T/R− spinal cord, F4/80 and Mac-3+ cells were poorly represented in those areas. This suggests that overlapping EAE was not taking place in those mice, and reinforces previous findings that T-cell infiltration precedes inflammatory response, characterized by the presence of macrophages.
An additional finding was that on SCI, T/R− mice displayed a much stronger and more diffuse expression of GFAP than T/R+ mice. GFAP expression correlates with astrocyte activation and gliosis. The presence of activated T cells in the spinal cord of T/R− mice may influence the activation process of astrocytes and other glial cells. Stab wounds have been shown to induce GFAP expression.34 Inflammation and trauma cause neuronal damage, which, in turn, triggers astrogliosis. Whether inflammation can cause astrogliosis independently of neuronal damage is unclear,35 although many cytokines can induce astrogliosis.35,36 In T/R− mice GFAP expression extended to spinal cord areas located far away from the stab wound, such as the lumbar region of the spinal cord, while GFAP expression in T/R+ animals was restricted to the area of the lesion. The remote astrogliotic response that we noticed in T/R− mice has been previously noticed in other clinical and experimental situations37 and could be caused by molecules diffused from the lesion site, or by products of lymphocytes present in other areas of the spinal cord. A more simplistic explanation would be that the remote gliosis is due to axonal transection caused by the injury and that the gliosis is a response to Wallerian degeneration of severed axons.38
The principle of neuroprotective autoimmunity has been applied to propose posttraumatic vaccination with weak, nonpathogenic autoantigens.39 The proposed treatment is based on beneficial effects caused by neuroantigen-specific T cells as described by Moalem and colleagues,40 Hauben and colleagues,41 Serpe and colleagues,42,43 and others. This concept has been recently challenged by Jones and colleagues,44 who described that myelin-reactive T cells caused intraspinal inflammation associated with functional impairment and exacerbation of posttraumatic lesion pathology. Our data with T/R− and T/R+ mice are in general agreement with that of Jones and colleagues.44 The discrepancies may indicate the different levels of tuning between aggression and regulation that exist in different situations.
Recently, Kipnis and colleagues45 showed that rats neonatally tolerized to myelin proteins exhibit worse retinal ganglion cell survival than nontolerant rats. Furthermore, elimination of CD4+CD25+ regulatory T cells led to enhanced neuronal survival on injury while administration of CD4+CD25+ regulatory T cells hampered neuronal survival. Thus, in these authors’ interpretation, regulatory T-cell activity navigates a compromise path between prevention of autoimmune disease (a function that Tregs are adept at) and tissue survival/maintenance (which Tregs supposedly hold back).45
In autoimmune demyelinating diseases of the CNS, T-cell-initiated demyelination leads to secondary axonal death. Regulatory T cells antagonize myelin-specific effector T cells (as well as other effector T cells) counteracting their effects.33 Based on the data shown here and our previously published data on the role of regulatory T cells in EAE, our interpretation of the role of regulatory T cells in SCI is different from the one proposed by Kipnis and colleagues at that time.45 Our interpretation is that regulatory T cells are beneficial, and it is based on the remarkable parallels between spontaneous EAE, a model in which MBP-specific T cells are undoubtedly pathogenic and regulatory T cells are undoubtedly protective, in EAE as in SCI. In the SCI model, the accumulation of CD4+3H12+ cells, as well as the down-modulation of surface expression of CD4 and TCR, were prevented by transferring CD4+ cells, and were also prevented when a small number of T cells expressing endogenous TCRs were naturally present, as in T/R+ mice. The down-modulation of CD4 surface expression in T/R− mice correlated with the generation of an encephalitogenic condition in spontaneous EAE, which was also prevented by the transfer of CD4+ cells before the development of disease. In fact, more recently, Kipnis and colleagues46 reported the possibility of a beneficial role for regulatory T cells, which happens in certain strains of mice due to a weakened specific T-cell response in the presence of regulatory cells. The authors suggest that the action of regulatory cells depends on interactions with microglia.
We consider that it is unlikely that very similar inflammatory reactions in which MBP-specific T cells play a major role would have opposite outcomes in terms of neuronal survival. However, we did not assess neuronal survival in the presence and absence of regulatory T cells in our SCI model. Hauben and Schwartz39 discussed the observation that EAE-resistant strains are better able to spontaneously manifest a beneficial, T-cell-dependent response to CNS injury, due to the absence of protective mechanisms in EAE-susceptible strains. In the mouse strains that we used (T/R+ versus T/R−), and very likely in other susceptible/resistant pairs, protective mechanisms are clearly dependent on the presence of CD4+ regulatory T cells. Our data, therefore, support a protective role for regulatory T cells in SCI, and are in agreement with data and discussions regarding susceptible and resistant strains,39,46 but not with data indicating that regulatory T cells antagonize protective autoimmunity.45 A balance must be reached between effector T cells and regulatory T cells, and, in our experimental model, absence of regulatory T cells leads to a harmful inflammatory response.
In conclusion, our experiments indicate that common elements participate in the regulation of SCI and spontaneous EAE. In T/R− mice, infiltrating MBP-specific CD4+ T cells that enter the CNS either as a consequence of SCI at young age, or later in life as spontaneous EAE develops, exhibit a similar phenotype of down-modulation of CD4 and, less markedly, the TCR. Moreover, in both models polyclonal CD4+ T cells, but no other T cells, can reduce the inflammatory load. Thus, the prevention of CNS tissue damage as a secondary effect of T-cell infiltration can be made by similar mechanisms in EAE model as well as in traumatic lesions of the spinal cord, and, possibly, in other inflammatory CNS pathologies.
Acknowledgments
We thank Drs. Eleanor Roberts and E.M. Burudi for critical review of the manuscript and Drs. Phillip Popovich and Steven J. Henriksen for helpful discussions.
Footnotes
Address reprint requests to Maria Cecilia G. Marcondes, Ph.D., The Scripps Research Institute, La Jolla, CA 92037. E-mail:cmarcond@scripps.edu or Juan J. Lafaille, Ph.D., Program of Molecular Pathogenesis, Skirball Institute for Biomolecular Medicine, New York University School of Medicine, New York, NY 10016. E-mail: lafaille@saturn.med.nyu.edu.
Supported by the Fundaçao de Amparo ‘a Pesquisa do Estado de Sao Paulo, Brazil (fellowship to M.C.G.M.); the National Multiple Sclerosis Society (to G.C.F. and J.J.L); the Christopher Reeve Paralysis Foundation (to J.J.L.); the National Institutes of Health/National Institute of Allergy and Infectious Diseases (to J.J.L.); and the Dana Foundation (to J.J.L.).
Present address of M.C.G.M.: Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA 92037.
Present address of G.C.F.: Immunobiology Center, Mount Sinai School of Medicine, New York, NY 10128.
Present address of H.S.F.: Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA 92037.
References
- Ghirnikar RS, Lee YL, Eng LF. Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res. 1998;23:329–340. doi: 10.1023/a:1022453332560. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, Veromaa T, Waisman A, Gaur A, Conlon P, Ling N, Fairchild PJ, Wraith DC, O’Garra A, Fathman CG, Steinman L. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- Haring JS, Pewe LL, Perlman S. Bystander CD8 T cell-mediated demyelination after viral infection of the central nervous system. J Immunol. 2002;169:1550–1555. doi: 10.4049/jimmunol.169.3.1550. [DOI] [PubMed] [Google Scholar]
- Haring JS, Perlman S. Bystander CD4 T cells do not mediate demyelination in mice infected with a neurotropic coronavirus. J Neuroimmunol. 2003;137:42–50. doi: 10.1016/S0165-5728(03)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Keere F, Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J Exp Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagomez D, Wensky AK, Wang Y, Lafaille JJ. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J Immunol. 2000;164:5499–5507. doi: 10.4049/jimmunol.164.10.5499. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Olivares-Villagomez D, Curotto de Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001;182:122–134. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin-2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B, Zhu J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J Neurobiol. 2005;62:207–218. doi: 10.1002/neu.20099. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- Forchheimer LL. Topical use of cortisone in sympathetic ophthalmia; four-year observation of a successfully treated case. Am J Ophthalmol. 1955;40:248–251. doi: 10.1016/0002-9394(55)91636-6. [DOI] [PubMed] [Google Scholar]
- Paez Allende F. Two cases of sympathetic ophthalmia cured with cortisone, hydrocortisone and broad spectrum antibiotics. Rev Assoc Med Argent. 1956;70:243–245. [PubMed] [Google Scholar]
- Olsson T, Diener P, Ljungdahl A, Hojeberg B, van der Meide PH, Kristensson K. Facial nerve transection causes expansion of myelin autoreactive T cells in regional lymph nodes and T cell homing to the facial nucleus. Autoimmunity. 1992;13:117–126. doi: 10.3109/08916939209001912. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Ebers GC, Johnson KP, Rodriguez M, Sibley WA, Wolinsky JS. The relationship of MS to physical trauma and psychological stress: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1999;52:1737–1745. doi: 10.1212/wnl.52.9.1737. [DOI] [PubMed] [Google Scholar]
- Acres RB, Conlon PJ, Mochizuki DY, Gallis B. Rapid phosphorylation and modulation of the T4 antigen on cloned helper T cells induced by phorbol myristate acetate or antigen. J Biol Chem. 1986;261:16210–16214. [PubMed] [Google Scholar]
- Pelchen-Matthews A, Parsons IJ, Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Thieme T, Vandenbark AA. Gangliosides induce selective modulation of CD4 from helper T lymphocytes. J Immunol. 1987;139:3295–3305. [PubMed] [Google Scholar]
- Davis CB, Littman DR. Disrupted development of thymocytes expressing a transgenic TCR upon CD4 overexpression. Int Immunol. 1995;7:1977–1986. doi: 10.1093/intimm/7.12.1977. [DOI] [PubMed] [Google Scholar]
- Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–778. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Chiu FC, Norton WT. Biochemical and immunocytochemical changes in glial fibrillary acidic protein after stab wounds. Brain Res. 1990;524:64–71. doi: 10.1016/0006-8993(90)90492-t. [DOI] [PubMed] [Google Scholar]
- Little AR, O’Callagha JP. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–618. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Walz W. Unusual topographical pattern of proximal astrogliosis around a cortical devascularizing lesion. J Neurosci Res. 2003;73:497–506. doi: 10.1002/jnr.10683. [DOI] [PubMed] [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Schwartz M. Therapeutic vaccination for spinal cord injury: helping the body to cure itself. Trends Pharmacol Sci. 2003;24:7–12. doi: 10.1016/s0165-6147(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]