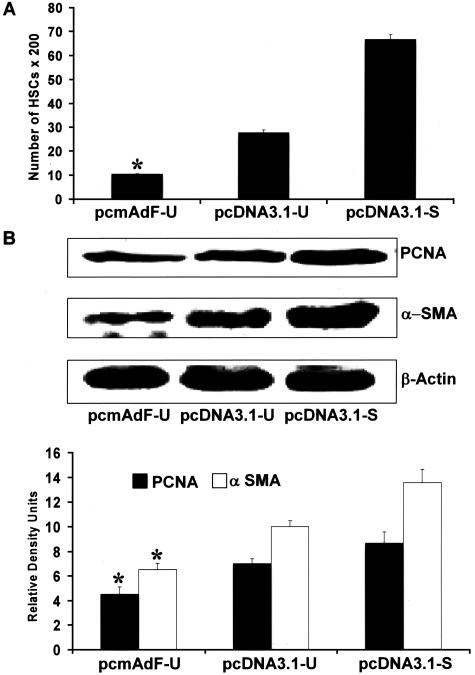
Figure 9.
Adiponectin overexpression in activated HSCs markedly diminishes activated HSC proliferation and α-SMA content. A: The adiponectin expression vector, pcmAdF (epitope encoding full-length adiponectin), was transfected as described in Figure 8 and found to reduce activated HSC BrdU incorporation. HSCs were cultured in a 96-well plate, 0.1 μg per well of plasmid DNA was transfected: pcmAdF (epitope encoding full-length adiponectin), pcDNA3.1-U (empty vector under SF conditions), or pcDNA3.1-S (empty vector in the presence of 10% FBS). BrdU was added after 24 hours of transfection and HSCs were allowed to grow for another 48 hours in the presence or absence of serum. Incorporation of BrdU into newly synthesized DNA was immunodetected as described in Materials and Methods and intensity of color complex was assayed spectrophotometrically. Calorimetric results were standardized to number of cells and plotted here as mean ± SE of three independent experiments performed in triplicate. Adiponectin overexpression markedly attenuated HSC proliferation to 10%, whereas the proliferation rate of HSCs transfected with vector control in the presence of 10% FBS (pcDNA3.1-S), was significantly higher. *P < 0.01, compared to untreated HSCs transfected with control vector (pcDNA3.1-U). B: Adiponectin vector transfection in SF-conditions (pcmAdF-U) in HSCs reduced protein expression levels of α-SMA and PCNA. To determine the effect of adiponectin on expression of α-SMA and PCNA, proliferation markers for HSCs, 4 μg of plasmid DNA, pcmAdF (epitope encoding full-length adiponectin), or pcDNA3.1 (vector control) were transfected into HSCs in 100-mm3 plates. After 48 hours, HSC lysates were prepared, total protein was quantified and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblot analysis with antibodies against α-SMA and PCNA. β-Actin served as a loading control. Shown is a representative immunoblot for the same along with densitometric analysis of the results.