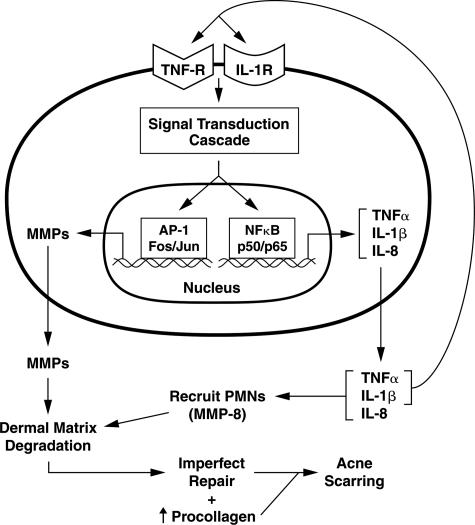
Figure 7.
Hypothetical model of the pathophysiology of inflammatory acne and dermal damage. In inflammatory acne lesions, NF-κB signaling is activated. As a consequence, NF-κB-driven inflammatory cytokine genes (eg, TNF-α and IL-1β) are induced. These primary cytokines will propagate the inflammatory response by acting on endothelial cells to elaborate adhesion molecules (eg, ICAM-1) to facilitate recruitment of inflammatory cells into the skin. TNF-α and IL-1β will also stimulate the production of secondary cytokines, such as IL-8, which can aid in chemotaxis of inflammatory cells. By working through their cell surface receptors, TNF-α and IL-1β not only amplify the NF-κB signaling cascade, but also activate MAP kinases to stimulate AP-1-mediated gene transcription. As a consequence of AP-1 activation (cJun induction), AP-1-driven MMPs are elaborated by resident skin cells. Along with MMP-8 and neutrophil elastase brought in by PMNs, they degrade the matrix. This is followed by matrix synthesis and repair, which is imperfect. Most of the imperfections would leave clinically undetectable deficits in the organization or composition, or both, of the extracellular matrix. However, when they occur to a significant extent throughout time, accompanied by sustained procollagen synthesis, acne scarring becomes clinically visible.