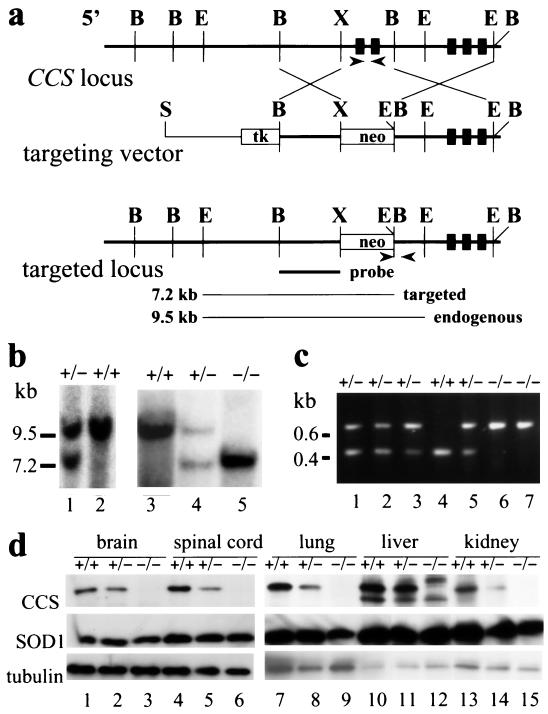
Figure 1.
Targeted disruption of the CCS gene by homologous recombination. (a) Maps of the wild-type CCS allele, the targeting vector, and the targeted CCS locus. Exons 1 to 5 of the CCS gene are denoted by black boxes. The targeting vector shows the replacement of exons 1and 2 and flanking genomic sequences including portions of the promoter by the neomycin gene (neo) and the HSV thymidine kinase gene (tk). Lines below indicate expected sizes from a Southern blot for EcoRI-digested fragments detected with a 5′-probe (black bar) from targeted and endogenous CCS alleles. B, BamHI; E, EcoRI; H, HindIII; S, SalI; X, XhoI. Arrows denote the sites within the targeted and wild-type CCS locus from which PCR primers were chosen for genotyping. (b) Analysis of genomic DNA from ES cells (lanes 1 and 2) and from progeny of CCS+/− crosses (lanes 3–5). Genotypes for the CCS targeted allele and the EcoRI fragments detected for endogenous (9.5 kb) and targeted (7.2 kb) CCS alleles with the 5′ probe are indicated. (c) PCR analysis of DNA extracted from tail clips. By using primers indicated in a, the 0.4-kb or 0.6-kb fragment is specific to the endogenous or targeted CCS allele respectively; wild-type (lane 4), heterozygous (lanes 1, 2, 3, and 5), and homozygous (lanes 6 and 7) CCS knockout mice are indicated. (d) Protein extracts (20 μg) from various tissues of wild-type (lanes 1, 4, 7, 10, and 13), heterozygous (lanes 2, 5, 8, 11, and 14), and homozygous (lanes 3, 6, 9, 12, and 15) CCS knockout mice were immunoblotted by using antisera specific for CCS, SOD1, and α-tubulin. Bound antibodies were detected by using an enhanced chemiluminescent detection method.