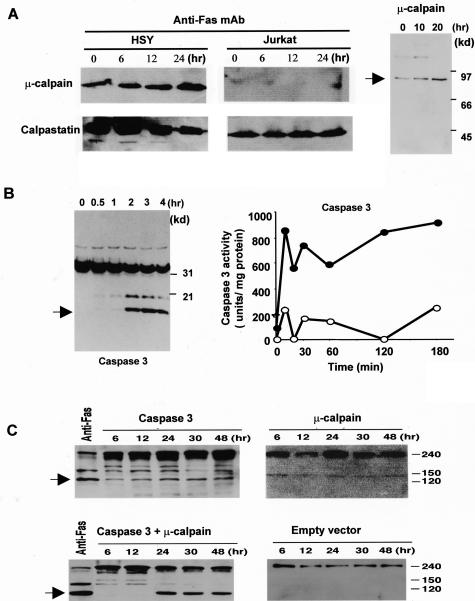
Figure 3.
Detection of cysteine proteases in anti-Fas-induced apoptotic HSY cells. Western blot analysis of μ-calpain, and calpastatin in apoptotic HSY and Jurkat cells stimulated with anti-Fas Ab (CH-11). A constitutive expression of μ-calpain, and its time-dependent increase were observed in anti-Fas-stimulated HSY cells. Calpastatin activity is shown to be constitutively expressed more than calpain expression and a time-dependent decrease of calpastatin expression was observed in apoptotic HSY cells, not in Jurkat cells. A: Western blot analysis of the active form of μ-calpain in apoptotic HSY cells stimulated with anti-Fas mAb (CH-11). B: Western blot analysis showing a time-dependent increase in caspase 3 and sequential activation of caspase 3-like protease in anti-Fas-induced apoptotic HSY cells. The caspase 3-like activity in the lysates (100 mg protein) (filled circle) or in the presence of 50 mmol/L MOCAc-DEVD-NH2 (open circle) was determined using fluorescent substrates in apoptotic HSY cells. One unit corresponds to the activity that cleaves 1 pmol of the respective fluorescent substrate at 30°C in 30 minutes. C: Detection of cleavage product of 120-kd α-fodrin in co-transfected HSY cells overexpressed with full-length caspase 3 and μ-calpain cDNAs. Analysis of lysates from caspase 3 and μ-calpain cDNA co-transfected cells revealed a fivefold to sevenfold increase of 120-kd α-fodrin in the level of expression of caspase 3 or μ-calpain in cells transfected with each construct.