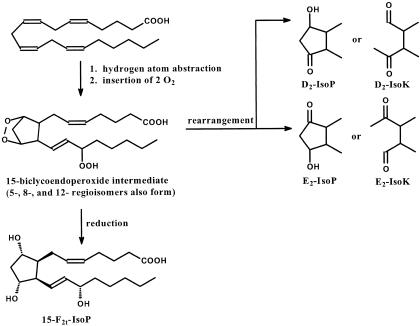
Figure 3.
Free radical-mediated hydrogen atom abstraction from AA followed by insertion of 2 O2 leads to generation of four regioisomeric endoperoxide intermediates, each of which may be reduced to form an F-ring or rearrange to form D-ring, E-ring, Tx-ring (not shown), or LG-like (IsoK) products. Similar reactions occur with DHA to generate NeuroPs and NeuroKs. Nomenclature for the IsoPs and NeuroPs follows conventions for PGs. Prostane rings are denoted D, E, F, or Tx and the subscripted number refers to the number of double bonds. The regioisomers are named by location of the side chain hydroxyl relative to the carboxyl. Default absolute configuration of side chain hydroxyl is S; R configuration is “epi” Side chains may be cis-or trans, denoted “c” or “t” in the subscript, with respect to cyclopentane ring hydroxyls. The structure of the most extensively studied IsoP, 15-F2t-IsoP, is shown.