Abstract
Wound-healing disorders are major complications of diabetes mellitus. Here, we investigated insulin-mediated signaling in nonwounded skin and in cutaneous tissue regeneration of healthy C57BL/6 and diabetes-impaired leptin-deficient obese/obese (ob/ob) mice. The insulin receptor (InsR) was abundantly expressed in wound margins and granulation tissue during acute healing in healthy mice. Remarkably, active signaling from the InsR, as assessed by phosphorylation of downstream targets such as protein tyrosine phosphatase-1B, glycogen synthase (GS), and GS kinase, was nearly absent in nonwounded and acutely healing skin from ob/ob mice. Systemic leptin administration to ob/ob mice reverted the diabetic phenotype and improved tissue regeneration as well as the impaired expression of InsR, insulin receptor substrate-1 and insulin receptor substrate-2, and downstream signaling (phosphorylation of GS kinase and GS) in late wounds and nonwounded skin of ob/ob mice. Importantly, tumor necrosis factor (TNF)-α was a mediator of insulin resistance in keratinocytes in vitro and in ob/ob wound tissue in vivo. Systemic administration of a monoclonal anti-TNF-α antibody (V1q) in wounded ob/ob mice attenuated wound inflammation, improved re-epithelialization, and restored InsR expression and signaling in wound tissue of ob/ob mice. These data suggest that InsR signaling in diabetes-impaired wounds is sensitive to inflam-matory conditions and that anti-inflammatory approaches, such as anti-TNF-α strategies, improve diabetic wound healing.
The functional connection between diabetes and foot ulceration was first recognized by the surgeon T.D. Pryce in 1887.1 For the first time, Pryce claimed in an article published in Lancet that “diabetes itself may play an active part in the causation of perforating ulcers.”1 Diabetic foot ulcers are skin lesions with a loss of epithelium that may extend into the dermis and may sometimes involve bone and muscle.2,3 It is now well established that ulcerations and subsequent amputation events of lower extremities represent serious complications of both types of diabetes mellitus and are associated with significant mortality.3,4 Thus, diabetic ulcers characterize an increasing clinical problem. The annual incidence of foot ulceration in the diabetic population is just over 2%,5,6 resulting in a lifetime risk of 15% for any diabetic patient to develop such a complication.7,8 Diabetic ulcers still have a poor prognosis, and the 3-year survival rates are between 50 and 59%, as assessed for Italy and Sweden, respectively.9,10 By contrast, the efforts to identify novel pharmacological approaches to improve significantly severe diabetes-impaired healing conditions have failed. Only recombinant platelet-derived growth factor (becaplermin) is now available for treatment of foot ulcers.11 Thus, Jeffcoate and Harding7 focus the challenge for future research in their review article on diabetic foot ulceration by their demand that “investment is urgently needed for basic research into the pathophysiology of chronic wounds.”
Here, we have used the obese/obese (ob/ob) mouse as a model system of diabetes-impaired wound healing. These mice are characterized by severe diabetes and obesity syndromes.12 The diseased phenotype is mediated by a functional loss of the ob gene, which normally encodes a 16-kd cytokine named leptin.13 Severely impaired wound-healing conditions in ob/ob mice were strongly improved by administration of leptin, where leptin mediated wound re-epithelialization in a direct manner but attenuated chronic wound inflammation in an indirect manner.14,15 In addition, systemic application of leptin to ob/ob mice also blunts both hyperglycemia and hyperinsulinemia and resolves the diabetic phenotype of the animals.14–16 It was reasonable to suggest that dysregulation and insensitivity of the insulin signaling machinery in resident skin cells might contribute to diabetes-impaired repair and that a leptin-driven adjustment of insulin sensitivity in skin tissue might be functionally connected to an improved healing in the animals. In line, skin keratinocytes have been shown to express the insulin receptor (InsR), which is functionally implicated in keratinocyte differentiation and glucose uptake.17,18
There is increasing evidence for a functional link between insulin resistance, obesity, and diabetes. Initial studies demonstrated an increase in adipocyte-derived tumor necrosis factor (TNF)-α in obese rodents that was functionally connected to insulin resistance.19 Interestingly, plasma TNF-α levels were also dependent on adipose tissue mass in humans,20,21 and clinical studies confirmed that the presence of inflammatory mediators predicts the development of type 2 diabetes mellitus.22–24 These observations suggest that obesity-associated inflammatory mediators such as TNF-α might contribute to insulin resistance in skin tissue. Using the leptin-deficient ob/ob mouse model, we investigated the insulin sensitivity of nonwounded and injured skin tissue under normal and diabetes-impaired conditions. Here, we provide evidence that disturbed insulin signaling pathways are associated with impaired repair in ob/ob mice and that TNF-α functionally interferes with insulin signaling and tissue regeneration at the wound site.
Materials and Methods
Animals
Female C57BL/6J (wild-type) and C57BL/6J-ob/ob mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and maintained under a 12-hour light/12-hour dark cycle at 22°C until they were 8 weeks of age. At this time, they were caged individually, monitored for body weight, and wounded as described below.
Treatment of Mice
Murine recombinant leptin (2 μg/g body weight) (Calbiochem, Bad Soden, Germany) and purified monoclonal anti-TNF-α antibody V1q25 (1 μg/g body weight) (Abcam Ltd., Cambridge, UK) were injected intraperitoneally in 0.5 ml of phosphate-buffered saline (PBS) for the indicated time periods. For local treatment, wounds of mice were covered with 1 μg of leptin in 20 μl of PBS twice a day (8:00 a.m. and 8:00 p.m.). Control mice were treated with PBS or an unspecific IgG (Santa Cruz, Heidelberg, Germany), respectively.
Wounding of Mice
Wounding of mice was performed as described previously.26,27 Briefly, mice were anesthetized with a single intraperitoneal injection of ketamine (80 mg/kg body weight)/xylazine (10 mg/kg body weight). The hair on the back of each mouse was cut, and the back was sub-sequently wiped with 70% ethanol. Six full-thickness wounds (5 mm in diameter, 3 to 4 mm apart) were made on the back of each mouse by excising the skin and the underlying panniculus carnosus. The wounds were allowed to form a scab. Skin biopsy specimens were obtained from the animals 1, 3, 5, 7, and 13 days after injury. At each time point, an area that included the scab, the complete epithelial and dermal compartments of the wound margins, the granulation tissue, and parts of the adjacent muscle and subcutaneous fat tissue was excised from each individual wound. As a control, a similar amount of skin was taken from the backs of nonwounded mice. For each experimental time point, tissue from four wounds each from four animals (n = 16 wounds, RNA analysis) and from two wounds each from four animals (n = 8 wounds, protein analysis) were combined and used for RNA and protein preparation. Nonwounded back skin from four animals served as a control. All animal experiments were performed according to the guidelines and approval of the local Ethics Animal Review Board.
RNA Isolation and RNase Protection Analysis
RNA isolation and RNase protection assays were performed as described previously.27,28 The cDNA probes were cloned using reverse transcriptase-polymerase chain reaction. The probes corresponded to nucleotides 3718 to 4080 (for InsR, NM010568.1), nucleotides 1173 to 1391 (for protein tyrosine phosphatase [PTP]-1B, BC010191.1), nucleotides 4261 to 4621 (for InsR substrate [IRS]-1, NM010570.2), nucleotides 1969 to 2201 (for IRS-2, AF090738), nucleotides 1294 to 1592 (for glucose transporter [Glut]-4, BC014282.1), nucleotides 796 to 1063 (for cyclooxygenase [COX]-2, M64291), nucleotides 541 to 814 (for TNF-α, NM013693), nucleotides 1405 to 1649 (for TNF-α R1, p55, NM001065.2), nucleotides 1192 to 1475 (for TNF-α R2, p75, NM001066.2), and nucleotides 163 to 317 (for GAPDH, NM002046).
Immunohistochemistry
Mice were wounded as described above. Animals were sacrificed at day 5 after injury. Complete wounds were isolated from the back, bisected, and frozen in tissue-freezing medium. Six-micrometer frozen sections were subsequently analyzed using immunohistochemistry as described previously.26 Additionally, wounds were fixed in formalin and embedded in paraffin. Paraffin-fixed sections were stained with hematoxylin and eosin. Antiserum against the β-subunit of InsR (Santa Cruz) was used for immunodetection.
Immunoblot Analysis
Wound, muscle, and liver tissue and cell culture lysates were prepared as described previously.26,29 Fifty micrograms of total protein lysate was separated using sodium dodecyl sulfate (SDS)-gel electrophoresis, and specific proteins were detected using antisera directed against InsRβ, Glut-4 (Santa Cruz), PTP-1B, IRS-1, IRS-2 (Biomol, Hamburg, Germany), phospho-glycogen synthase kinase (GSK)-3α/β, phospho-glycogen synthase (GS), phospho-tyrosine (Cell Signaling, Frankfurt, Germany), or actin (Sigma, Deisenhofen, Germany).
Determination of Phospho-InsRβ by Immunoprecipitation
Five hundred micrograms of total wound protein lysate was incubated overnight with 2.5 μg of a monoclonal, biotinylated anti-phospho-tyrosine (4G10) antibody (Biomol). Next, 150 μg of streptavidin-coupled magnetic beads (MyOne; Dynal, Hamburg, Germany) was added for 2 hours. Magnetic beads were isolated and washed with PBS, and protein was eluted using Laemmli buffer.
Determination of Glucose Uptake
Quiescent, confluent human HaCaT keratinocytes were incubated in 35-mm wells with 1 ml of Krebs-Ringer solution (Sigma, Deisenhofen, Germany) in the presence or absence of insulin (0.1 and 1 μg/ml) for 20 minutes. Subsequently, 0.5 μCi of d-[2-H3]glucose (Amersham, Freiburg, Germany) was added per 35-mm well. Cells were harvested using 10% (w/v) SDS after different time points of incubation.
Enzyme-Linked Immunosorbent Assay
Total wound lysate was analyzed for the presence of immunoreactive TNF-α by enzyme-linked immunosorbent assay (ELISA) using the Quantikine murine ELISA kit (R&D Systems, Wiesbaden, Germany).
Determination of Blood Glucose, Insulin, and Leptin Levels
Blood glucose levels were determined using the Accutrend sensor (Roche Biochemicals, Mannheim, Germany). Serum insulin and leptin were analyzed by ELISA (Crystal Chemicals, Chicago, IL) as described by the manufacturer.
Determination of Cell Viability
Viability of cultured keratinocytes was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay following a published protocol30 and the lactate dehydrogenase assay. The lactate dehydrogenase assay was performed according to the instructions of the manufacturer (Roche Biochemicals, Mannheim, Germany).
Statistical Analysis
Data are shown as means ± SD. Data analysis was performed using the unpaired Student’s t-test with raw data. Statistical comparison between more than two groups was performed by analysis of variance (Dunnett’s method).
Results
Insulin Signaling Molecules in Skin and Wound Tissue of Healthy and Diabetic Mice
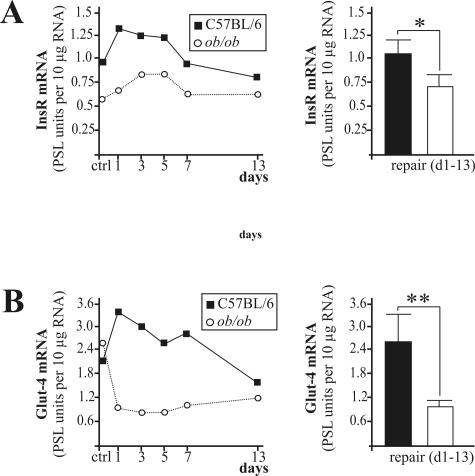
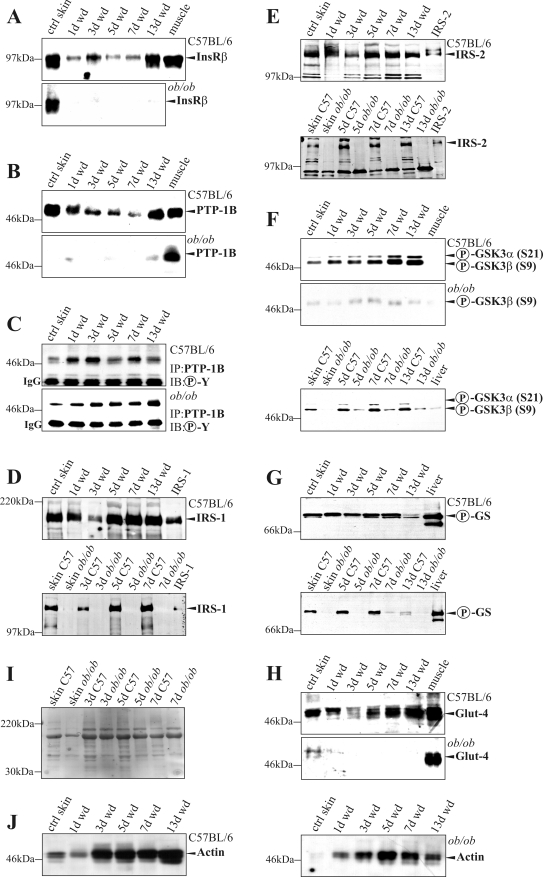
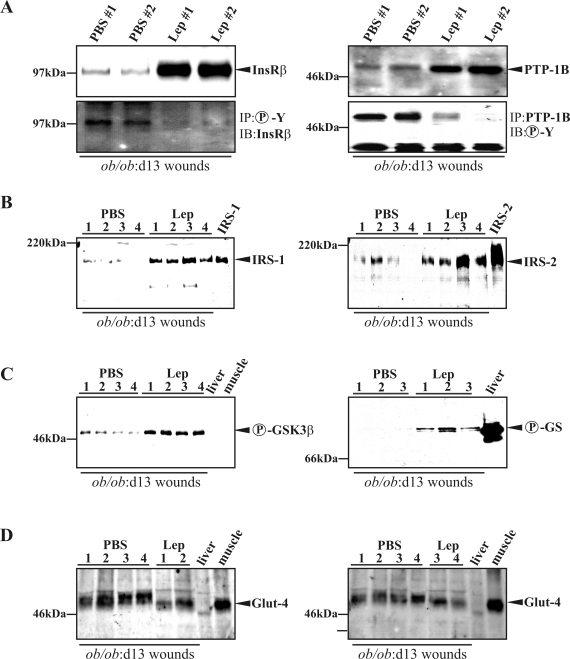
In this study, we investigated the availability of insulin signaling pathways in skin of normal and diabetes-impaired wound healing. The ob/ob mouse is characterized by a severe type 2 diabetes mellitus12 and suffers from disturbed wound-healing conditions.14,15,31 We found that expression of InsR (Figure 1A) and Glut-4 (Figure 1B) mRNA was significantly reduced in impaired wounds of diabetic ob/ob mice when compared with acutely healing wounds of C57BL/6 mice. By contrast, mRNA expression levels did not markedly change during healing in both groups for PTP-1B, IRS-1, and IRS-2 (data not shown). However, protein expression revealed marked differences in the availability and activation of insulin-sensitive components in skin tissue of control and diseased mice. In clear contrast to healthy mice, the constitutively expressed InsR completely diminished on injury in diabetes-impaired wound tissue (Figure 2A). Notably, InsR and PTP-1B expression during normal healing paralleled a reduced amount of protein during the acute repair process (Figure 2, A and B, top panels, 1 day wound to 7 days wound). However, PTP-1B was barely detectable in ob/ob mice (Figure 2B, bottom panel). PTP-1B represents a central negative regulator of insulin action32 that is inactivated by tyrosine phosphorylation.33 Interestingly, we observed an increase of tyrosine phosphorylated and thus inactive PTP-1B (Figure 2C, top panel, 1 day wd to 7 days wd) that paralleled InsR down-regulation (Figure 2A) during acute healing in control mice. Nevertheless, the small amounts of PTP-1B in wounds of ob/ob mice were most likely inactive, because tyrosine phosphorylation of PTP-1B increased during repair (Figure 2C, bottom panel). We also found the expression of InsR adaptor molecules IRS-1 (Figure 2D) and IRS-2 (Figure 2E) to be markedly reduced in skin and wounds of diabetic ob/ob mice. Finally, we investigated possible consequences of altered InsR, PTP-1B, or IRS-1 and -2 availability during diabetes-impaired repair. To this end, we determined activation of the key down-stream molecules GSK3α and -β and GS in normal and wounded skin. Insulin causes inactivation of GSK3 as a result of serine phosphorylation of the kinase, finally leading to serine dephosphorylation and functional activation of GS.34 We observed increasing amounts of phosphorylated GSK3α (Ser21) and GSK3β (Ser9) during normal repair, which were nearly completely absent in ob/ob mice (Figure 2F). However, although wound GSK3α/β activity was most likely blunted by serine phosphorylation, we recognized a marked phosphorylation (Ser641) of GS during the complete healing period in healthy mice, which did not persist in diseased animals (Figure 2G). Moreover, Glut-4 was constitutively expressed during normal repair but was strongly down-regulated in diabetes-impaired wound conditions (Figure 2H).
Figure 1.
Expression of insulin signaling molecules in skin repair. Regulation of InsR (A) and Glut-4 (B) mRNA expression in nonwounded back skin (ctrl) and wound tissue isolated from wild-type (C57BL/6) and ob/ob mice. The time after injury is indicated. Each single experimental time point represents 16 wounds (n = 16) isolated from four individual mice of two experimental series. Mean expression levels during repair are shown in the right panels. **, P < 0.01; *, P < 0.05. Bars indicate the mean ± SD over the healing period (days 1 to 13) from 80 wounds from 20 animals.
Figure 2.
Key proteins of the insulin signaling cascade during normal and diabetes-impaired wound (wd) healing. Immunoblots showing InsR (A), PTP-1B (B), tyrosine-phosphorylated (Y-P) PTP-1B (C), IRS-1 (D), IRS-2 (E), phosphorylated (S21, S9) GSK3α/β (F), phosphorylated (S641) GS (G), and Glut-4 (H) in nonwounded (ctrl skin) and wounded skin in C57BL/6 and ob/ob mice as indicated. A control for equal loading (Ponceau S staining) is shown in I. J: Integrity of protein lysates is again controlled by immunodetection of actin. The time after injury is indicated. Each time point depicts eight wounds (n = 8) from four individual mice (n = 4). Liver and muscle tissue was used as control tissue to prove specificity of antibodies.
Localization of Insulin-Responsive Cells in Normal Wound Tissue
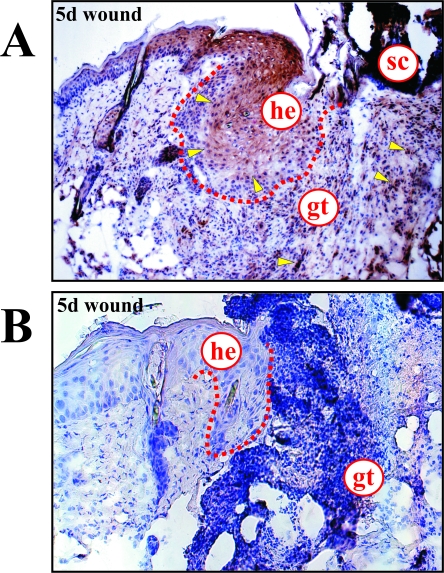
Immunohistochemistry revealed the InsR to be expressed in proliferating keratinocytes located at the wound margins and within the granulation tissue in 5-day wound tissue of healthy mice (Figure 3A), indicating that both epidermal and mesenchymal wound cells were potentially sensitive toward insulin. Notably, we could barely detect InsR-specific signals in wound sections isolated from diabetic ob/ob mice (Figure 3B), which were characterized by clearly reduced hyperproliferative epithelia located at the margins of the wound.14
Figure 3.
Induction and localization of the InsR at the wound site. Immunohistochemical localization of InsR protein in 5-day wounds of healthy C57BL/6 (A) and diabetic ob/ob mice (B). Particularly strong signals for InsR expression are indicated by arrows. he, hyperproliferative epithelium; gt, granulation tissue; sc, scab.
Systemic Leptin Administration Resolves the Diabetic Phenotype, Improves Skin Repair, and Adjusts Disturbed Wound Insulin Signaling Pathways in ob/ob Mice
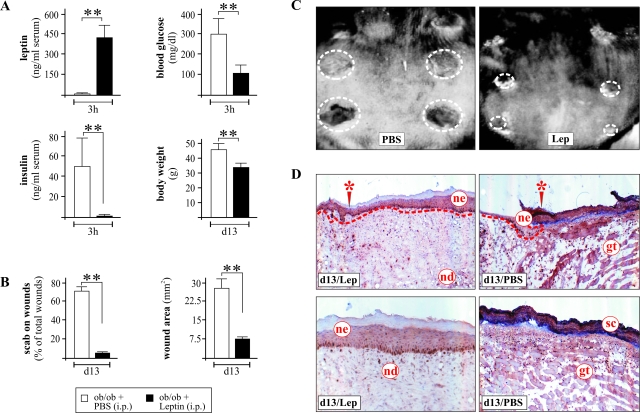
It is well established that systemic treatment of leptin-deficient ob/ob mice with recombinant leptin results in resolution of both the diabetic and impaired healing phenotype.14,16 Thus, ob/ob mice were injected intraperitoneally with recombinant leptin (2 μg/g body weight, once a day) for 13 days. As shown in Figure 4A, we found high serum leptin levels 3 hours after injection. The diabetic phenotype of leptin-injected ob/ob mice was resolved, because hyperinsulinemia and blood glucose were rapidly adjusted to normal. Moreover, after 13 days of leptin treatment, mice revealed a significant loss of body weight (Figure 4A) and an improved healing, as assessed by the loss of scabs after wound re-epithelialization and reduction of wound areas (Figure 4, B and C). Histological analysis of 13-day wound tissue convincingly demonstrated the potency of systemically administered leptin to improve wound re-epithelialization in ob/ob mice (Figure 4D). Late wound areas from leptin-treated mice were characterized by a robust formation of a multilayered and organized neo-epidermis and neo-dermis, which completely covered the site of injury (Figure 4D, left panels). By contrast, wounds of PBS-treated mice revealed only small and reduced neo-epithelia at the wound margins and completely failed to cover the site of injury with a well-developed granulation tissue and neo-epithelium. At this stage of impaired healing, wound coverage was represented not by cells but by a robust scab (Figure 4D, right panels).
Figure 4.
Administered leptin is biologically active. A: Blood leptin, insulin, and glucose levels 3 hours after systemic application of recombinant leptin. The ob/ob mice were treated with leptin for 13 days, after which the body weight of the animals was monitored. B: Presence of scab-covered wounds and wound area after 13-day treatment with PBS or leptin. **, P < 0.01 as compared with PBS-treated animals. Bars indicate the means ± SD from nine individual animals (n = 9). C: Photographs of 13-day wounds in PBS- or leptin-treated ob/ob mice. D: Representative histological analysis of a 13-day wound tissue of a leptin-treated (left panels) or PBS-treated (right panels) ob/ob mouse. Top panels show the wound margin area (site of initial injury is marked by an asterisk); bottom panels show the middle of the wound. gt, granulation tissue; nd, neo-dermis; ne, neo-epidermis; sc, scab.
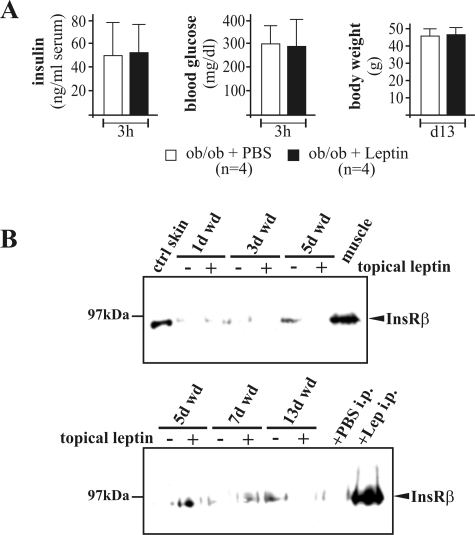
Next, we investigated the presence and activation of key insulin signaling molecules in late chronic and improved wounds (day 13 after wounding) isolated from PBS- or leptin-treated ob/ob mice. We could not detect significant changes in total InsR, PTP-1B, IRS-1, and Glut-4 mRNA expression in improved and diabetes-impaired wounds, but IRS-2 mRNA levels were significantly increased in impaired wound tissue (data not shown). However, chronic diabetes-impaired wounds in PBS-injected ob/ob mice were characterized by low protein expression levels of InsR and its negative regulator PTP-1B32 (Figure 5A). Interestingly, PTP-1B was highly phosphorylated in chronic wounds from PBS-treated mice and thus most likely is in an inactive state33 (Figure 5A, right panels). This observation was functionally supported by a strong phosphorylation of the InsR, which was only detectable in the presence of phosphorylated PTP-1B in impaired wound tissue of PBS-treated mice (Figure 5A, left panel). Moreover, the InsR adaptor molecules IRS-1 and -2 were expressed in wounds after leptin administration but were absent in chronic healing conditions (Figure 5B). Additionally, we found GSK3β (Ser9) (Figure 5C, left panel) and the GS (Figure 5C, right panel) to be phosphorylated after leptin treatment. Finally, Glut-4 protein was expressed in both normal and impaired repair; however, the protein isolated from leptin-treated improved wounds appeared to be slightly different in terms of its migratory behavior in SDS gels (Figure 5D). It is noteworthy that the effects of leptin on the insulin signaling cascade were restricted to its systemic properties. We tested the potency of topically applied leptin to amend the disturbed insulin cascade in wounds of ob/ob mice. In the absence of systemic short-term (blood insulin and glucose levels) and long-term (body weight) effects of topically applied leptin (Figure 6A), we could not detect any re-increase in InsR expression in wounds of topically treated animals (Figure 6B).
Figure 5.
Disturbed key components of insulin signaling are adjusted by systemic leptin administration in wounds of diabetic ob/ob mice. Immunoblots showing InsR, phospho-InsR, PTP-1B, and phospho-PTP-1B (A); IRS-1 and IRS-2 (B); phospho-GSK3α/β and phospho-GS (C); and Glut-4 (D) in 13-day wounds of PBS- and leptin-treated ob/ob mice as indicated. Numbers indicate individual mice. Each time point depicts eight wounds (n = 8) from four individual mice (n = 4). Liver and muscle tissue and lysates from IRS-1- and IRS-2-overexpressing cells were used as controls to demonstrate specificity of antibodies.
Figure 6.
Topical treatment of wounds (wd) with leptin did not improve InsR expression. A: Blood insulin and glucose levels 3 hours after topical application of recombinant leptin (1 μg/wound). After 13 days of leptin treatment, the body weight of the animals was monitored. Bars indicate the means ± SD from four individual animals (n = 4). B: Immunoblots showing InsR expression in nonwounded skin (ctrl skin) and wounds of topically treated ob/ob mice as indicated. Each time point depicts eight wounds (n = 8) from four individual mice (n = 4). Thirteen-day wound tissue from leptin-injected mice and muscle tissue served as positive controls.
Leptin Improves Sensitivity of the Insulin Signaling Cascade in Nonwounded Skin of Diabetic ob/ob Mice
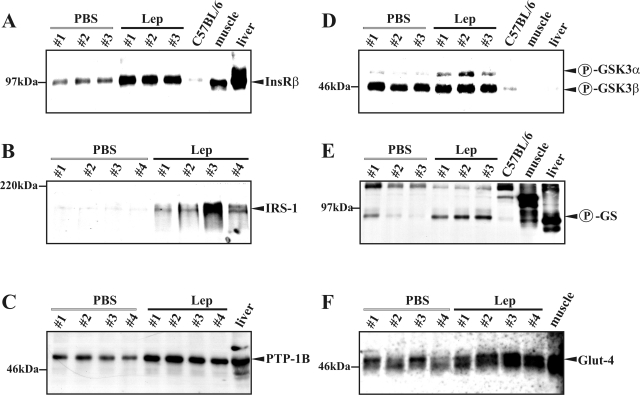
As a next step, we determined expression and activation of key molecules of the insulin pathway in nonwounded skin of PBS- and leptin-treated ob/ob mice. We did so to clarify whether the above-mentioned alterations in insulin sensitivity were restricted to a disturbed healing or a common disturbance in skin tissue of these diabetic mice. Protein expression of the InsR (Figure 7A), IRS-1 (Figure 7B), PTP-1B (Figure 7C), and Glut-4 (Figure 7F) and phosphorylation of GSK3α (Figure 7D) and the GS (Figure 7E) were increased in nonwounded skin tissue on leptin treatment. These data clearly indicate disturbed insulin sensitivity in skin tissue before wounding, which is most likely transferred into the impaired healing process.
Figure 7.
Leptin adjusts key molecules of insulin signaling in nonwounded diabetic skin tissue. ob/ob mice were systemically treated with PBS or leptin (Lep) for 7 days. Immunoblots showing InsR (A), IRS-1 (B), PTP-1B (C), phospho-GSK3α/β (D), phospho-GS (E), and Glut-4 (F) in nonwounded skin of PBS- and leptin-treated ob/ob mice as indicated. Numbers indicate individual mice. Skin of C57BL/6 mice, or liver and muscle tissue were used as controls.
Chronic Insulin and TNF-α Exposure Might Contribute to Impaired Insulin Sensitivity in Keratinocytes
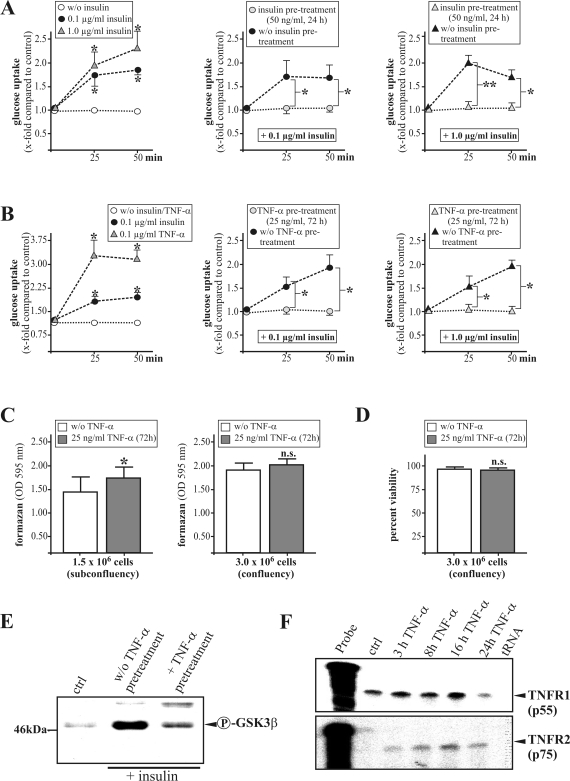
Finally, we aimed to identify possible mediators that might be responsible for the systemic effects of leptin on insulin sensitivity in diabetic wound tissue. Because ob/ob mice suffer from hyperinsulinemia (Refs. 12 and 16; this study), we hypothesized that chronic exposure of resident skin cells to insulin might contribute to insulin insensitivity. Pretreatment (24 hours) of human HaCaT keratinocytes with low amounts of insulin (50 ng/ml) completely abolished the observed dose-dependent glucose uptake into the cells after acute insulin stimulation (0.1 and 1.0 μg/ml) (Figure 8A).
Figure 8.
The role of chronic insulin and TNF-α exposure for insulin actions in cultured keratinocytes. Serum-starved HaCaT keratinocytes were stimulated for glucose uptake (x-fold compared with control) in the presence or absence of chronic insulin (A) or TNF-α (B) pretreatment as indicated. **, P < 0.01; *, P < 0.05 as compared with controls. Bars indicate the means ± SD obtained from four independent cell culture experiments (n = 4). Keratinocyte viability as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (C) or lactate dehydrogenase (D) assay in the presence or absence of a 72-hour TNF-α stimulation as indicated. E: Immunoblot demonstrating phospho-GSK3β on insulin stimulation in the presence or absence of TNF-α pretreatment. F: RNase protection assay demonstrating mRNA expression of TNF-α receptor 1 (TNFR1, p55) and TNF-α receptor 2 (TNFR2, p75) in starved HaCaT keratinocytes on TNF-α (2 nmol/L) stimulation as indicated. Hybridization against tRNA was used as a negative control.
Additionally, chronic release of TNF-α from adipose tissue has been shown to functionally contribute to systemic insulin resistance in obese rodents.19 Although an acute stimulation of HaCaT keratinocytes by TNF-α strongly increased glucose uptake into the cells, we observed a complete inhibition of insulin-mediated glucose uptake into keratinocytes after chronic TNF-α pretreatment (25 ng/ml, 72 hours) of the cells (Figure 8B). Here, it is important to note that chronic exposure of keratinocytes to TNF-α did not reduce the viability of cells (Figure 8, C and D). Moreover, exposure to TNF-α inhibited insulin-stimulated serine phosphorylation of the GSK3β isoform in the cells (Figure 8E). Interestingly, HaCaT keratinocytes expressed both p55 and p75 TNF-α receptors (TNFRs), although only the p75 receptor was induced by its own ligand (Figure 8F).
Systemic Neutralization of TNF-α Attenuated Wound Inflammation and Strongly Improved Wound Morphology in Diabetic ob/ob Mice
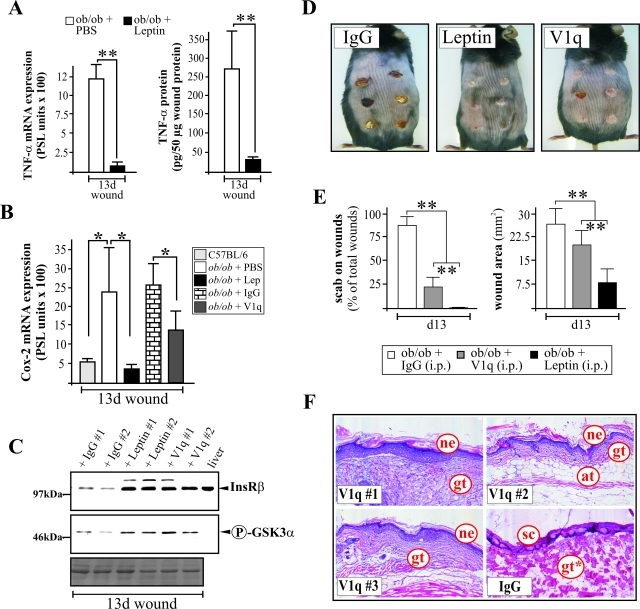
Our in vitro observations provided strong evidence that TNF-α might also represent an inhibitor of insulin action in skin cells, because ob/ob mice were characterized by increased levels of circulating TNF-α.19 To confirm a possible functional connection between elevated TNF-α levels, wound insulin resistance and diabetes-disturbed wound morphology in ob/ob mice in vivo, we treated the animals at days 9 and 12 after wounding with a systemic application of the monoclonal anti-TNF-α antibody V1q.25 Remarkably, the above-mentioned leptin-mediated improvement of insulin signaling was clearly associated with a strong attenuation of TNF-α expression at the wound site (Figure 9A). More importantly, only two systemic applications of the anti-TNF-α antibody V1q into ob/ob mice resulted in a significant attenuation of wound inflammation as assessed by the expression of COX-2 mRNA at the wound site (Figure 9B). It is important to note that even a short-term systemic neutralization of TNF-α by V1q in ob/ob mice restored the expression of the InsR and phosphorylation of GSK3α at the wound site in vivo to levels comparable with leptin treatment (Figure 9C). Moreover, V1q treatment improved diabetes-disturbed wound healing in ob/ob mice as shown by scab-free wound tissue (Figure 9, D and E, left panel) and re-epithelialized wounds associated with reduced wound areas (IgG, 26.8 ± 5.5 mm2, versus V1q, 19.6 ± 3.9 mm2, P < 0.001; n = 18 wounds) (Figure 9, D and E). Moreover, wounds from V1q-administered ob/ob mice were characterized by the strong formation of granulation tissues covered by organized and robust neo-epithelia (Figure 9F). Here, it is noteworthy that untreated control animals (IgG) completely failed to form an epithelial tongue at this stage of healing (Figure 9F; see also PBS-treated control mice in Figure 4D).
Figure 9.
Systemic neutralization of TNF-α strongly improves diabetes-impaired wound healing. A: Quantification of TNF-α mRNA (RNase protection assay; left panel) and protein (ELISA; right panel) expression in 13-day wounds from PBS- and leptin (Lep)-treated ob/ob mice. **, P < 0.01 as indicated by the brackets. Bars indicate the means ± SD obtained from 18 wounds (n = 18) isolated from nine animals (n = 9). B: COX-2 mRNA expression in 13-day wound tissue from ob/ob mice that have been treated with PBS, leptin, unspecific control antibody (IgG), or a monoclonal anti-TNF-α antibody (V1q) as indicated. *, P < 0.05 as indicated by brackets. Bars indicate the means ± SD obtained from wounds (n = 16) isolated from four different animals (n = 4). C: Immunoblot showing the expression of InsR and phospho-GSK3α in 13-day wounds from IgG-, leptin-, or anti-TNF-α antibody (V1q)-injected ob/ob mice as indicated. Ponceau S staining of the blot is shown as a loading control. D: Photographs of 13-day wounds in IgG-, leptin-, and anti-TNF-α (V1q)-treated ob/ob mice. E: Presence of scab-covered wounds and wound area of 13-day wounds in IgG-, leptin-, and anti-TNF-α (V1q)-treated ob/ob mice. **, P < 0.01 as indicated by the brackets. Bars indicate the means ± SD from 18 wounds (n = 18) from three individual animals (n = 3). F: Histological analyses of 13-day wound tissue isolated from three (n = 3) individual anti-TNF-α (V1q)- or IgG-treated ob/ob mice as indicated. Formalin-fixed, paraffin-embedded 6-μm sections were counterstained using hematoxylin and eosin. at, adipose tissue; gt, granulation tissue; gt*, atrophied gt; ne, neo-epidermis, sc, scab.
Discussion
Diabetes mellitus currently develops into one of the main threats of human health with an explosive increase in the number of people diagnosed with diabetes worldwide.35 Visceral obesity is now well established to represent one of the predominant risk factors in the development of an insulin resistance.35 One major complication of the diabetic disease is the ulceration of the foot.2,3 Although diabetic ulcerations are of clinical importance, nearly all promising applications of recombinant factors, with the exception of platelet-derived growth factor-BB,11 failed to be transferred into the human system. Thus, it is reasonable to emphasize that we need a fundamental understanding of the pathophysiology of diabetic wounds to specifically identify future therapeutic strategies.
A novel approach to expand our understanding of pathomechanisms underlying diabetic ulcerations might come from important findings that functionally connect insulin resistance in liver and muscle tissue to elevated serum levels of adipocyte-derived TNF-α in obese rodents. Even more important, systemic neutralization of TNF-α improved peripheral uptake of glucose in response to insulin in these obese animals.19 Here, it is worthy to note that human adipose tissue has also been shown to constitutively express TNF-α,20 and that there is strong evidence for a positive correlation between body mass index and circulating TNF-α levels in humans21 and the development of type 2 diabetes dependent on inflammatory parameters.22–24 Thus, it was now reasonable to hypothesize that diabetes-impaired wound healing in obese mice might be characterized by the failure of resident cells at the wound site to respond properly to an insulin stimulus. Moreover, we postulated that a potential insulin resistance of injured skin tissue, and thus comparable with systemic insulin insensitivity, might be functionally connected to increased obesity-mediated TNF-α production.19
In line with this hypothesis, we first recognized that regulation of key molecules of the insulin signaling cascade were disturbed in nonwounded and injured skin of diabetic mice. Interestingly, marked changes occurred at the protein level on injury, because we found a robust constitutive mRNA expression for central proteins (eg, InsR, PTP-1B, IRS-1, and IRS-2), indicating that expressional regulation most likely occurred from stably transcribed mRNA pools. Additionally, because wound tissue is characterized by a dynamic infiltration of cells, there is evidence that cell influx also contributes to differential changes in total wound mRNA and protein expression. The diabetes-associated expressional down-regulation of InsR, as the primary sensor for insulin at the wound site, appeared especially to represent one major event in decreasing peripheral insulin sensitivity at sites of ongoing wound inflammation in these animals.15,36 The loss of a functional InsR might counterregulate glucose utilization by resident skin cells to break the vicious circle of energy production by fermentation that acidifies the chronic wound microenvironment under prolonged inflammatory conditions. In good accordance with this hypothesis, we found that chronic exposure of TNF-α was able to inhibit insulin-stimulated glucose uptake into keratinocytes, thus contributing to the above-mentioned effect by a desensitization of cells toward an insulin signal.
It became obvious that impaired wounds in ob/ob mice were characterized by the nearly complete absence of InsR expression, which was normally expressed at the wound margins and within the granulation tissue in healthy animals. Interestingly, the residual InsR protein in chronic wound tissue was strongly phosphorylated at tyrosine residues. The overall low abundance of the InsR in diabetes-impaired wounds was in close functional connection to reduced amounts of PTP-1B, representing a negative regulator of InsR activation in vivo.33 Phosphorylation of PTP-1B leads to its enzymatic inactivation.33 As a consequence, we found highly phosphorylated InsR molecules in diabetes-impaired wound tissues. This observation might reflect a compensatory mechanism of disturbed wound tissue to allow residual signaling from markedly reduced InsR molecules. However, the remaining InsR appeared to be functionally inactive, because effective signaling from the phosphorylated InsR remained severely disturbed. The consequences of these findings include the presence of resident cells in nonwounded skin and chronic wounds of diabetic mice that are most likely disturbed in terms of sensitivity toward external signals. This observation might explain, at least partially, that topical application of growth factors into chronic wound tissue has failed to improve wound healing to date. Obviously, resident cells could not respond properly to insulin because their signaling machinery is severely disturbed, an effect that might also hold true for other factors. Keratinocyte behavior is especially influenced by the presence of an undisturbed insulin signaling. Lack of InsR in keratinocytes led to an abrogation of glucose uptake and an impairment of differentiation.17,18 Additionally, these findings clearly impact nonwounded diabetic skin that was characterized by a markedly reduced expression of InsR.
Here, we could show that systemic leptin administration to ob/ob mice resolved the diabetic phenotype, attenuated wound inflammation and TNF-α expression (Goren et al15; this study), and improved skin repair and insulin sensitivity. Notably, topical administration of small amounts of leptin (1 μg/wound) failed to influence systemic parameters such as serum insulin (this study) and glucose (Bing et al31; this study) as well as corticosterone levels.31 Because we could not observe restored InsR expression after topical treatment, it is tempting to argue that leptin-mediated improvement of insulin sensitivity and healing of wounds pivotally involves systemic mechanisms.
Nevertheless, the central question still remained whether obesity and insulin resistance of resident wound cells might be functionally connected by TNF-α. To finally determine a functional role for TNF-α in this process, we have treated animals with the monoclonal antibody V1q, which has been shown to efficiently neutralize TNF-α in mice in vivo.25 Treatment was initiated from day 7 after wounding, because we had to avoid interference with physiologically elevated levels of TNF-α during acute wound inflammation.15 It is important to note here that anti-TNF-α treatment strongly improved the disturbed wound-healing conditions in ob/ob mice. Wounds of anti-TNF-α-treated ob/ob mice revealed an attenuated wound inflammation associated with improved wound contraction and granulation tissue formation as well as a complete re-epithelialization. Moreover, this process was paralleled by the strong re-appearance of InsR expression in wounds of ob/ob mice. Thus, our findings strongly suggest that systemic TNF-α levels were functionally connected to impaired healing conditions under diabetic and obese conditions in ob/ob mice. Additionally, our data provide strong evidence that anti-inflammatory pharmacological strategies to interfere with potentially elevated levels of inflammatory mediators such as TNF-α might restore insulin sensitivity in diabetes-impaired wounds and thus might represent a novel therapeutic approach to improve healing.
In summary, we have shown that key components of the insulin signaling machinery were dysregulated in nonwounded and injured skin of diabetic ob/ob mice. Systemic administration of leptin normalized these impaired conditions. However, leptin action on insulin signaling was indirect and partially related to an attenuation of chronic inflammatory conditions. Moreover, our data provide evidence that functional signaling from InsR participates in skin repair. InsR signaling in diabetes-impaired wounds appeared to be sensitive to inflammatory conditions, suggesting that anti-inflammatory approaches, such as anti-TNF-α strategies, could improve diabetic wound healing.
Acknowledgments
We thank Dr. M. Kock and Dr. A. Theissen for their help with the animal experiments.
Footnotes
Address reprint requests to Dr. Stefan Frank, pharmazentrum frankfurt/ZAFES, Institut für Allgemeine Pharmakologie und Toxikologie, Klinikum der JW Goethe-Universität Frankfurt am Main, Theodor-Stern-Kai 7, D-60590 Frankfurt am Main, Germany. E-mail: s.frank@em.uni-frankfurt.de.
Supported by the Deutsche Forschungsgemeinschaft (SFB 553, grant FR 1540/1-1, and grant FR 1540/2-1).
References
- Pryce TD. A case of perforating ulcers of both feet associated with diabetes and ataxic symptoms. Lancet. 1887;2:11–12. [Google Scholar]
- Boulton AJM. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47:1343–1353. doi: 10.1007/s00125-004-1463-y. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Ledoux WR. Epidemiology of diabetic foot ulcers and amputations: evidence for prevention. Williams R, Herman W, AL Kinmonth, Wareham NJ, editors. Chichester: UK, Wiley; The Evidence Base for Diabetes Care. 2002:pp 641–665. [Google Scholar]
- Carrington AL, Abbott CA, Griffiths J, Jackson N, van Ross ERE, Boulton AJM. A foot care program for diabetic unilateral amputees. Diabetes Care. 2001;24:216–221. doi: 10.2337/diacare.24.2.216. [DOI] [PubMed] [Google Scholar]
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabetic Med. 2002;20:377–384. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- Muller IS, de Grauw WJ, van Gerwen WH, Bartelink ML, van Den Hoogen HJ, Rutten GE. Foot ulceration and lower limb amputation in type 2 diabetic patients in Dutch primary health care. Diabetes Care. 2002;25:570–574. doi: 10.2337/diacare.25.3.570. [DOI] [PubMed] [Google Scholar]
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg. 1998;176:5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233:485–491. doi: 10.1111/j.1365-2796.1993.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rats in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5 year follow-up. Diabetes Care. 2001;24:78–83. doi: 10.2337/diacare.24.1.78. [DOI] [PubMed] [Google Scholar]
- Wieman TJ, Smiell JM, Su Y. Efficacy and safety of topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren I, Kämpfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes. 2003;52:2821–2832. doi: 10.2337/diabetes.52.11.2821. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Wertheimer E, Spravchikov N, Trebicz M, Gartsbein M, Accili D, Avinoah I, Nofeh-Moses S, Sizyakov G, Tennenbaum T. The regulation of skin proliferation and differentiation in the IR null mouse: implications for skin complications of diabetes. Endocrinology. 2001;142:1234–1241. doi: 10.1210/endo.142.3.7988. [DOI] [PubMed] [Google Scholar]
- Spravchikov N, Sizyakov G, Gartsbein M, Accili D, Tennenbaum T, Wertheimer E. Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes. 2001;50:1627–1635. doi: 10.2337/diabetes.50.7.1627. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue: regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-α system in humans. J Clin Endocrinol Metab. 1997;82:3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities Study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J, Frank S. the function of nitric oxide in wound repair: inhibition of inducible nitric oxide-synthase severely impairs wound reepithelialization. J Invest Dermatol. 1999;113:1090–1098. doi: 10.1046/j.1523-1747.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999;13:2002–2014. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Kämpfer H, Kalina U, Mühl H, Pfeilschifter J, Frank S. Counterregulation of interleukin-18 mRNA and protein expression during cutaneous wound repair in mice. J Invest Dermatol. 1999;113:369–374. doi: 10.1046/j.1523-1747.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ring BD, Scully S, Corrine CR, Baker MB, Cullen MJ, Pelleymounter MA, Danilenko DM. Systemically and topically administered leptin both accelerate wound healing in diabetic ob/ob mice. Endocrinology. 2000;141:446–449. doi: 10.1210/endo.141.1.7373. [DOI] [PubMed] [Google Scholar]
- Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- Tao J, Malbon CC, Wang HY. Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J Biol Chem. 2001;276:29520–29525. doi: 10.1074/jbc.M103721200. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Albert KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–252. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]