Abstract
Paraplegia resulting from ischemia is a catastrophic complication of thoracoabdominal aortic surgery. The current study was designed to investigate the effects of diazoxide (DZ) on mitochondrial structure, neurological function, DNA damage-repair, and apoptosis in spinal cord ischemia-reperfusion injury. Rabbits were subjected to 30 minutes of spinal cord ischemia and reperfusion (1 hour) with or without diazoxide (n = 6 in each group) by clamping and releasing the infrarenal aorta. The neurological functional score was significantly improved in the DZ-treated ischemia-reperfusion injury group. Electron microscopic studies demonstrated that mitochondrial damage in the spinal cord after injury was significantly reduced by DZ. Mitochondrial superoxide and hydrogen peroxide levels were also markedly decreased in the DZ-treated injury group compared with the untreated group. DZ decreased levels of the oxidative DNA damage product 8-oxoG and increased levels of the DNA repair enzyme OGG-1. Furthermore, DZ inhibited apoptosis via caspase-dependent and -independent pathways. These studies indicate for the first time that the mitochondrial K-ATP channel opener diazoxide improves neurological function after spinal cord ischemia and reperfusion by diminishing levels of reactive oxygen species, decreasing DNA oxidative damage, and inhibiting caspase-dependent and -independent apoptotic pathways while preserving mitochondrial structure.
Paraplegia may complicate repairs of extensive thoracabdominal aortic aneurysms as a result of ischemic injury to the spinal cord that results from interruption of intercostal and lumbar arterial circulation.1–5 Clinical adjuncts designed to reduce ischemia times, monitor neurological function, and allow for swelling of the spinal cord postoperatively have improved outcomes but do not eliminate the problem.6–10 Whereas complete postoperative neurological deficits were the rule in the past, today vascular surgeons are encountering more delayed and partial neurological deficits as a result of refinements in surgical technique. These types of neurological deficits result from ischemia and reperfusion injury and may potentially be ameliorated by pharmacological manipulation of this process. For this reason, our interest has centered on the basic mechanisms of injury of nervous tissue caused by ischemia and reperfusion.
Important mechanisms in ischemia-reperfusion injury in neural tissue include glutamate receptor-mediated excitotoxicity, generation of reactive oxygen species (ROS), calcium influx, loss of membrane potential, mitochondrial failure, and apoptosis.11–13 Mitochondrial DNA is particularly sensitive to ischemia-reperfusion injury, and mitochondria play a central role in cell death signaling by releasing proteins that activate downstream phases of apoptosis. These proteins include cytochrome c, Smac, apoptosis-inducing factor (AIF), and endonuclease G.14
Prevention of mitochondrial failure improves cellular survival after an ischemic event. This phenomenon is seen in ischemic preconditioning, a process that involves mitochondrial K-ATP channels.15 Pharmacological agents that maintain mitochondrial K-ATP channels can prevent mitochondrial failure in a manner similar to that induced by ischemic preconditioning.16–19 Diazoxide (DZ), a specific opener of the mitochondrial membrane K-ATP channel, has been found to limit ischemia-reperfusion injury and apoptosis in a number of in vivo and in vitro models.16,20–24
It has been shown in a rabbit model of spinal cord injury that diazoxide can ameliorate the effect of a temporary ischemic insult to the spinal cord.25 In a previous study, we have demonstrated that a 30-minute period of aortic occlusion led to activation of caspase-3, oxi-dative damage to DNA, the activation and exhaustion of DNA repair enzymes, and apoptosis as demonstrated by transferase-mediated dUTP nick-end labeling (TUNEL).26 We hypothesized that mitochondrial K-ATP channel openers would prevent the accumulation of ROS and ameliorate the downstream effects that lead to apoptosis. The studies we report here document the beneficial clinical effect of diazoxide in this model through its effect on the production of reactive oxygen species in the mitochondria, the reduction of oxidative damage to DNA, the increased activity of the DNA repair enzyme OGG-1, the structural integrity of mitochondria, and the occurrence of apoptosis in spinal cord cells. Thus, diazoxide ameliorates the downstream apoptotic effects of mitochondrial injury while sustaining the activity of the DNA repair enzyme OGG-1 and the structural integrity of mitochondria.
Materials and Methods
Surgical Procedure
In a protocol approved by our Animal Care and Use Committee, New Zealand White rabbits weighing approximately 3 kg were administered a combination of intravenous medazalam (0.25 mg/kg), metomidine (0.05 mg/kg), atropine (0.04 mg/kg), and a bolus of propofol (2 mg/kg) to induce anesthesia and were sustained with a propofol drip (2 mg/kg/minute) through an ear vein. The contralateral auricular artery was cannulated, and the arterial pressure monitored by transducer. Sterile instruments and surgical techniques were used for all animals that were not sacrificed immediately postoperatively. The aorta was exposed through a small flank incision, the peritoneum was reflected anteriorly, and the aorta was identified and clamped just distal to the left renal artery. After 30 minutes of occlusion time, the clamp was removed, and the incision was closed in two layers. Animals were closely observed in the surgical suite. Postoperative analgesia was maintained with intramuscular injections of banamine (3 mg/kg).
Animals receiving DZ were given an intravenous dose of 5 mg/kg 10 minutes before application of the infrarenal aortic clamp. Hypotension produced by DZ or by release of the aortic clamp was corrected by saline boluses in 50-ml increments. Mean arterial blood pressure was always maintained between 60 and 80 mmHg.
The rabbits were sacrificed with lethal intravenous pentathol injections after 1 hour of reperfusion for biochemical studies or after 2 days for functional studies. For the biochemical studies, the aortic arch was rapidly exposed by sternotomy, the descending thoracic aorta was cannulated with intravenous tubing, and 1 L of saline at 4°C was infused. Outflow was achieved by opening the right atrium. Rapid laminectomy was performed from the cervical to the sacral vertebrae. Samples of the lumbar spinal cord were harvested at least 2 cm distal to the 12th rib to avoid contamination by nonischemic tissue. Samples of the thoracic cord were harvested above the 12th rib to serve as nonischemic controls. Samples were snap frozen, formalin fixed, or prepared for electron microscopy study as described. Spinal cord tissue (L4 level) was used for electron microscopy study and immunohistochemical sampling. The rest of the lumbar spinal cord was used for ROS generation study and Western blot analysis.
Preparation of Mitochondria
Rabbit spinal cord mitochondria were isolated from the cortex of New Zealand White rabbits using a Percoll gradient method described by Sims27 with minor modifications. The isolation buffer contained 225 mmol/L mannitol, 75 mmol/L sucrose, 0.5 mmol/L ethylenediamine tetraacetic acid (EDTA), 5 mmol/L HEPES, and 1 mg/ml fatty acid-free bovine serum albumin (BSA), pH adjusted to 7.3 with KOH. Spinal cord tissue was homogenized using a glass/glass homogenizer in isolation buffer containing 12% Percoll and carefully layered on the top of a 12%/24%/42% discontinuous gradient of Percoll. After 11 minutes of centrifugation at 31,000 × g, the mitochondrial fraction was collected from the top of the 42% Percoll layer of the gradient and then washed twice. For the final wash, we used isolation buffer with BSA omitted and the EDTA concentration reduced to 0.1 mmol/L. All isolation procedures were performed at 0 to 2°C. During experimentation, mitochondria were stored on ice at a final concentration of 15 to 20 mg protein/ml in isolation medium until use. The protein concentration in each preparation was determined by the Biurett method using a plate reader.
Neurological Assessment
Neurological status was scored by assessment of hindlimb neurological function at 1 hour after the reperfusion procedure. Animals were classified on a 5-point scale according to the methods of Johnson et al28: 0, hindlimb paralysis, no voluntary hindlimb movement; 1, severe paraparesis, movement of joints perceptible; 2, functional movement, no hop, active movement but unable to sit without assistance; 3, ataxia, able to sit but disconjugate hop; 4, minimal ataxia and weak hop; and 5, normal neurological function and complete recovery of hindlimb function. The neurofunctional score was evaluated nonparametrically.
Transmission Electron Microscopy
For electron microscopy study, thin sections of ventral horn were cut from Epon tissue blocks, fixed in 2% glutaraldehyde, and subjected to postfixation with 1% osmium tetroxide in 0.1 mol/L cacodylate. Formvar-coated copper grids were stained with 2% uranyl acetate, which was followed by subsequent dehydration with series of ethanol. The samples were embedded overnight with a 1:1 ratio of propylene oxide to epoxy resin. Thereafter, this solution was replaced with 100% epoxy resin and then polymerized in a dry oven at 60°C. Ultrathin sections (70 nm thick) from the embedded samples were imaged by use of a Philips (Bothell, WA) CM 120 transmission electron microscope. Mitochondria number and mitochondria area were determined, and more than 100 mitochondria were examined under each experimental condition. The percentages of mitochondrial membrane damage and mitochondrial swelling were determined by methods described previously.12
Chemiluminescent Measurement of Superoxide and Hydrogen Peroxide (H2O2) Production by Intact Mitochondria
Bis(N-methylacridinium) (lucigenin) and 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) were purchased from Sigma (St. Louis, MO). Lucigenin- or luminol-derived chemiluminescence was monitored with a Berthold Biolumat LB 9505 at 37°C. For measurement of lucigenin-derived chemiluminescence, the reaction mixture contained 0.1 mg of mitochondrial protein in 1 ml of air-saturated respiration buffer in the presence of exogenous substrate (6 mmol/L succinate) with or without the pharmacological uncoupling agent carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (Sigma).29 The respiration buffer contained 70 mmol/L sucrose, 220 mmol/L mannitol, 2 mmol/L HEPES, 2.5 mmol/L KH2PO4, 2.5 mmol/L MgCl2, 0.5 mmol/L EDTA, and 0.1% BSA, pH 7.4. The lucigenin-derived chemiluminescence was initiated by adding 5 μmol/L lucigenin and was continuously monitored for 40 to 60 minutes. This is a non-redox-cycling concentration of lucigenin.29 For measurement of luminol-derived chemiluminescence, the reaction mixture contained 0.1 mg of mitochondrial protein in 1 ml of air-saturated respiration buffer plus succinate (6 mmol/L) and horseradish peroxidase (10 μg/ml). The luminol-derived chemiluminescence was initiated by adding 10 μmol/L luminol and was continuously monitored for 40 to 60 minutes.
Determination of 8-OxoG Generation
8-Oxo-7,8-dihydrodeoxyguanine (8-oxoG) expression in spinal cord tissue was determined as we previously described.30 Briefly, the slides were fixed and washed with phosphate-buffered saline (PBS), covered by anti-8-oxoG antibody overnight at 4°C. The slides were stained with diaminobenzamide tetrahydrochloride and counterstained with Methyl Green. The slides were mounted with appropriate mounting media.
Western Blot Analysis
Western blot analysis (wb) was performed as we previously described.26,31 Tissue samples were homogenized in a lysis buffer (0.1 mol/L NaCl, 0.01 mol/L Tris-HCl, pH 7.5, 1 mmol/L EDTA, and 1 μg/ml aprotinin), and then the homogenates were centrifuged at 7000 × g for 15 minutes at 4°C. Supernatants were used as protein samples. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed in a 10% polyacrylamide gel under nonreducing conditions. In brief, protein samples were boiled at 100°C in 2.5% SDS and 5% β-mercaptoethanol, and lysates equivalent to 20 μg of protein from each sample were run on the gel for 90 minutes at 20 mA together with a size marker (rainbow-colored protein; Amersham). The electrophoresis running buffer contained 25 mmol/L Tris base, 250 mmol/L glycine, and 0.1% SDS. Proteins on the gel were transferred to a nitrocellulose membrane with a transfer buffer that consisted of 48 mmol/L Tris base, 39 mmol/L glycine, 0.4% SDS, and 20% methanol. After transfer, membranes were placed in 1% powdered milk in PBS to block nonspecific binding. After reacting with the primary and secondary antibodies, the membrane was subjected to the Enhanced Chemiluminescence analysis system from Amersham. Polyclonal antibody against OGG1 was obtained from Novus Biological (Littleton, CO). Monoclonal antibodies against actin (Ab-6; Oncogene Research Products, MA) were used as controls for equal protein loading. To ascertain specific binding of the antibody for the protein, another membrane was stained without the primary antibody.
Immunohistochemical Staining
Immunohistochemical staining was performed as we previously described.26,31 Briefly, after samples were deparaffinized, spinal cord sections were rinsed in 0.1 mol/L PBS for 20 minutes and blocked in 2% normal horse serum for 2 hours. The sections were then incubated with primary antibody OGG1 (1:100; Novus), caspase-3 (1:100; EMD Biosciences, San Diego, CA), AIF (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), or polyadenosine-ribose polymerase (PARP-1) (1:100; TM of Trevigen, Gaithersburg, MD) in 10% normal horse serum or 10% normal rabbit serum and 0.3% Triton-X 100 for 20 hours at 4°C. After endogenous peroxidase activity was quenched by exposure of the slides to 0.3% H2O2 and 10% methanol for 20 minutes, the slides were washed in PBS and incubated with secondary antibody-horseradish peroxidase conjugate (Tago, Burlingame, CA). The final reaction was achieved by incubating the sections with freshly prepared reagent containing 3-amino-9-ethylcarbazole (Sigma) dissolved in dimethyl-formamide and sodium acetate. The sections were counterstained with hematoxylin, mounted, and reviewed with an Olympus microscope. Two trained independent observers reviewed these sections. Sample identities were concealed during scoring, and at least three samples were scored per group. The presence of positive immunohistochemical staining was assessed by microscopic examination of the final slides and evaluated by percentage of positive staining in the ventral areas of the spinal cord (0 to 100%). The specificity of positive staining was further confirmed by substitution of normal rabbit serum instead of primary antiserum.
Determination of OGG1 Activity
The activities of DNA repair enzymes in spinal cord tissue were determined by gel-retardation binding assay using A/8-oxoG containing DNA.32 Oligonucleotide substrates (20 nucleotides) were synthesized and constructed as follows: A, A/G 5′-CCGAGGAATTAGCCTTCTG-3′ and 3′-GCTCCTTAAGCGGAAGACG-5′; B, A/GO 5′-CCGAGGAATTAGCCTTCTG-3′ and 3′-GCTCCTTAAOCGGAAGACG-5′ (O for 8-oxoG); C, C/GO 5′-CCGAGGAATTCGCCTTCTG-3′ and 3′-GCTCCTTAAOCGGAAGACG-5′; and D, C/G 5′-CCGAGGAATTCGCCTTCTG-3′ and 3′-GCTCCTTAAGCGGAAGACG-5′.
Duplex A to C contains an A/G, A/8-oxoG, and C/8-oxoG base-base mismatches, respectively, whereas duplex D is a homoduplex containing no mismatch. These oligonucleotides can be uniquely labeled at the 5′ end with polynucleotide kinase and [γ-32P]ATP or labeled at the 3′ end with Klenow fragment and [α-32P]dCTP.
OGG1 glycosylase activity was performed according to Hazra et al,33 except that excess DNA substrates containing A/8-oxoG mismatches were added. A 25-μl DNA binding reaction contained 20 μg of protein, 25 mmol/L HEPES (pH 7.6), 50 mmol/L KCl, 2.5 mmol/L EDTA, 2 mmol/L dithiothreitol, and 2.5% glycerol. Two picomoles of unlabeled DNA substrates containing A/8-oxoG mismatches was added before adding 10 fmol of C/8-oxoG-containing DNA labeled at the 8-oxoG strand. The reactions were incubated at 37°C for 1 hour, terminated by phenol-chloroform extraction and ethanol precipitation. Samples were dissolved in 3 μl of sequencing dye. After heating at 90°C for 3 minutes, samples were resolved on a 14% polyacrylamide-8.3 mol/L urea DNA sequencing gel and analyzed by autoradiography.
To Determine Apoptosis and Apoptosis Pathways
To detect DNA fragmentation in situ, nick-end labeling (TUNEL staining) was performed according to our previously reported method30 using an ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD). Briefly, the nuclei of the tissue sections were stripped of proteins by incubating with 20 μg/ml proteinase K for 10 minutes after deparaffinization. After treating with 0.3% H2O2 in distilled water for 5 minutes, the sections were mixed with terminal deoxynucleotidyl transferase buffer (30 mmol/L Tris, pH 7.2, 140 mmol/L sodium cacodylate, and 1 mmol/L cobalt chloride; Boehringer Mannheim, Indianapolis, IN) containing terminal deoxynucleotidyl transferase enzyme (0.5 U/ml; Boehringer Mannheim) and biotin-16-dUTP (0.04 mmol/L; Boehringer Mannheim) containing 30 mmol/L cobalt chloride and incubated in a humidified chamber at 37°C for 120 minutes. The reaction was terminated by incubating with 300 mmol/L NaCl and 30 mmol/L sodium citrate for 15 minutes at 25°C. After washing with 50 mmol/L Tris-HCl, pH 7.7, sections were developed with diaminobenzadine/H2O2 solution. Counterstaining was performed with hematoxylin. After three washes in Tris-HCl, pH 7.7, the sections were dehydrated in ascending ethanol series. After immersion in xylene, the sections were coverslipped in Permount. To determine the number of motor neurons that underwent apoptosis, the positive or negative motor neurons in the TUNEL staining were counted. The caspase-dependent (caspase-3) and caspase-independent (AIF and PARP) apoptotic pathways were investigated by immunohistochemical staining for appropriate proteins as described above.
Statistical Analysis
Results of the quantitative studies are expressed as means ± SEM. Statistical comparisons within each group were performed by using analysis of variance for repeated measures followed by Fisher’s least-significant difference test of repeated measures when appropriate. Comparisons between groups were performed by using factorial analysis of variance followed by Fisher’s least-significant difference test of repeated measures. We analyzed the neurological score nonparametrically by using an unpaired Student’s t-test. Statistical significance was accepted at P < 0.05.
Results
Clinical Outcome
Table 1 indicates systolic and diastolic arterial pressures in both groups at baseline, ischemia, and reperfusion interval. There were no significant differences between groups at those intervals. Mean arterial blood pressure was always maintained between 60 and 80 mmHg.
Table 1.
Systolic and Diastolic Arterial Pressures in Both Groups at Baseline, Ischemia, and Reperfusion Interval
| SCI group (mmHg)
|
SCI+DZ group (mmHg)
|
|||
|---|---|---|---|---|
| Systolic | Diastolic | Systolic | Diastolic | |
| Baseline | 79 ± 5 | 46 ± 5 | 84 ± 4 | 54 ± 3 |
| Ischemia | 82 ± 10 | 60 ± 7 | 79 ± 8 | 51 ± 9 |
| Reperfusion | 75 ± 3 | 52 ± 3 | 75 ± 7 | 50 ± 8 |
SCI, spinal cord ischemia.
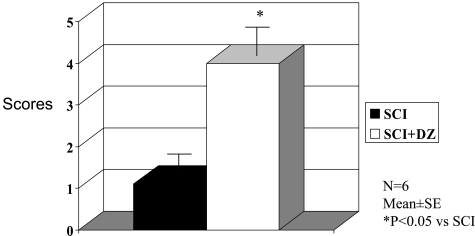
Thirty minutes of ischemia caused by infra-renal aortic occlusion resulted in immediate hind limb paralysis in all control animals. There was no improvement in function over 48 hours, and further follow-up was curtailed for humane reasons. Treatment with DZ improved function dramatically because all rabbits were able to use their hind legs well, although they did not maintain the ability to hop (Figure 1).
Figure 1.
Effect of mitochondrial K-ATP channel opener DZ on neurological functional scores after spinal cord ischemia and reperfusion injury. The neurological function was assessed 1 hour after starting reperfusion. SCI, spinal cord ischemia.
Mitochondrial Structure
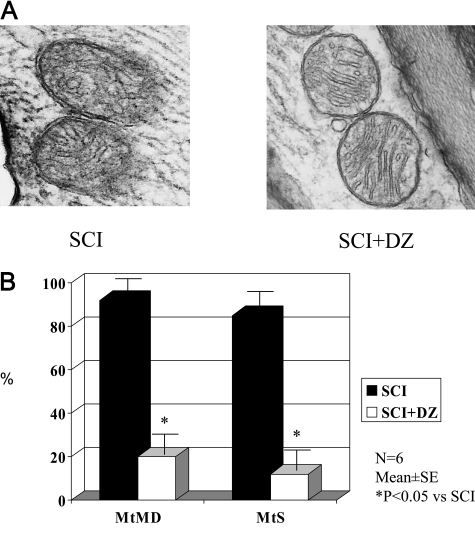
Electron microscopic study demonstrated that the mitochondria from the ischemic lumbar spinal cords studied as soon as 1 hour after reperfusion showed swelling, poorly oriented cristae, and gaps in the mitochondrial membrane (Figure 2A). By contrast, mitochondria from rabbits treated with DZ appeared markedly better with less mitochondrial membrane damage and swelling in the rabbit spinal cord after ischemia and reperfusion (Figure 2B).
Figure 2.
A: The effect of mitochondrial K-ATP channel opener DZ on mitochondrial structure in rabbit spinal cord after ischemia-reperfusion injury (magnification, ×12,500). SCI, spinal cord ischemia. B: The effect of mitochondrial K-ATP channel opener DZ on mitochondrial membrane damage (MtMD, %) and mitochondrial swollen (MtS, %) in rabbit spinal cord after ischemia-reperfusion injury.
ROS Generation in Mitochondria
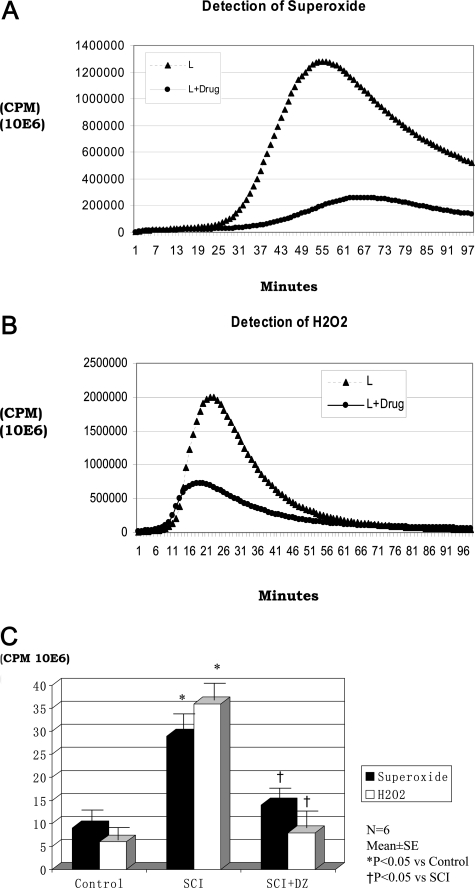
The mitochondria isolated immediately from the lumbar spinal cords of untreated rabbit displayed high levels of superoxide generation compared with thoracic mitochondria of treated animals (Figure 3A). Similarly, mitochondrial fractions from the ischemic reperfused lumbar spinal cord contained more H2O2 (Figure 3B). Total superoxide and hydrogen peroxide (the areas under the curves) measured from these experiments are shown in Figure 3C. Mitochondrial ROS production in lumbar spinal cord of animals with ischemia-reperfusion injury was significantly increased compared with that of the control mitochondria from the thoracic spinal cord in these animals. Treatment with DZ before occlusion reduced the generation of ROS (superoxide and H2O2) to near nonischemic or control levels.
Figure 3.
A: Mitochondrial superoxide generation in rabbit lumbar (L) spinal cord after ischemia-reperfusion injury in the absence (L) or presence (L+drug) of DZ. B: Mitochondria H2O2 generation in rabbit lumbar (L) spinal cord after ischemia-reperfusion injury in the absence (L) or presence (L+drug) of diazoxide. C: ROS generation (area under the cover) in rabbit spinal cord of three groups. The thoracic spinal cord (n = 6; three animals from spinal cord ischemia [SCI] group and three animals from DZ-treated SCI group) was used for control group in this figure and in Figures 4 to 7. In all of the animals, the thoracic spinal cord is intact after spinal cord ischemia. There are no differences in findings in the thoracic spinal cord between SCI and SCI+DZ groups.
Prevention of Irreparable Oxidative DNA Damage
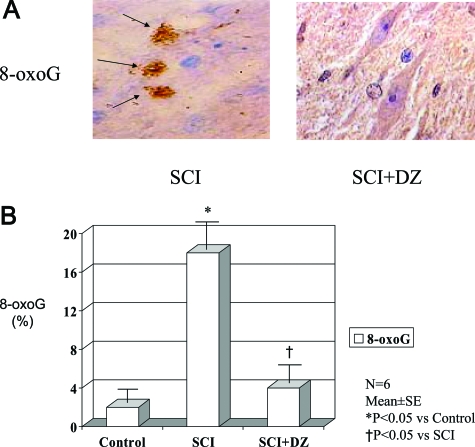
Sections of spinal cords from ischemic and reperfused lumbar areas were stained for the most common and stable product of oxidized DNA, 8-oxoG (Figure 4A). Cells staining positively were scarce in the DZ-treated animals but were abundant in the untreated animals (4 versus 17%; Figure 4B).
Figure 4.
A: Oxidative DNA damage (8-oxoG) staining (arrows) in rabbit spinal cord after ischemia-reperfusion injury in the absence (SCI) or presence (SCI+DZ) of diazoxide (magnification ×1000). B: Oxidative DNA damage (8-oxoG) level in rabbit spinal cord of three groups.
Expression and Activity of DNA Repair Enzyme OGG1
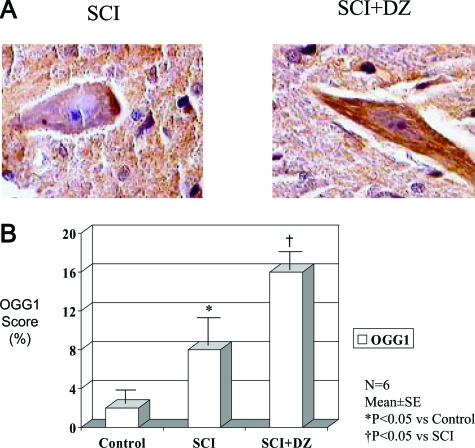
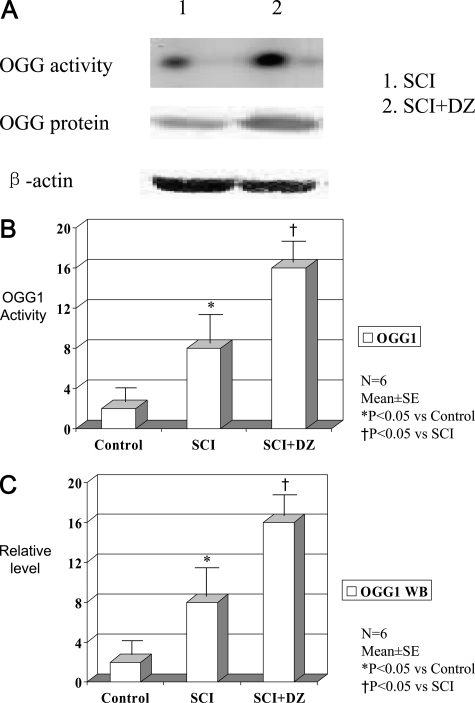
The expression of OGG1 was determined by immunohistochemical staining (Figure 5A) in the motor neuron region. The OGG1 staining score was significantly increased in animals treated with DZ compared with untreated animals (Figure 5B). Furthermore, WB studies showed that the spinal cords in the treated animals had sustained the protein concentration and activity of DNA repair enzyme OGG1 compared with untreated animals (Figure 6).
Figure 5.
A: Immunohistochemical staining of DNA repair enzyme OGG1 in rabbit spinal cord after ischemia-reperfusion injury in the absence (SCI) or presence (SCI+DZ) of diazoxide (magnification, ×1000). B: Immunohistochemical staining scores of DNA repair enzyme OGG1 in rabbit spinal cord of three groups.
Figure 6.
A: The banding activity and protein level (Western blot analysis) of DNA repair enzyme OGG1 in rabbit spinal cord after ischemia-reperfusion injury in the absence (SCI) or presence (SCI+DZ) of diazoxide. B: DNA repair enzyme OGG1 banding activity in rabbit spinal cord of three groups. C: Western blot analysis of OGG1 in rabbit spinal cord of three groups.
Prevention of Apoptosis and Cell Death
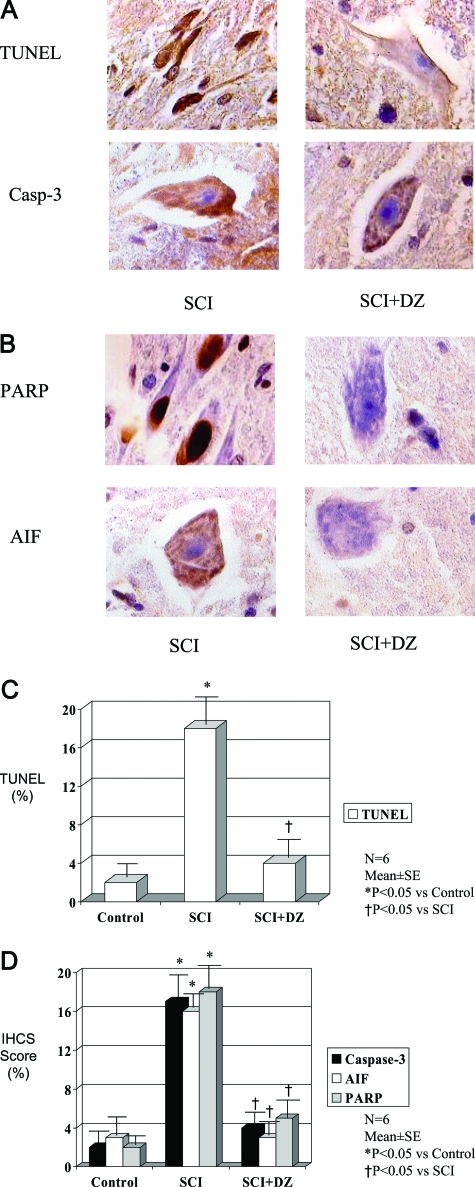
Treatment with DZ reduced greatly the numbers of cells staining for caspase 3, AIF, and TUNEL while virtually eliminating the profound staining for PARP-1 found in the nuclei of the lumbar spinal cord of the untreated animals (Figure 7).
Figure 7.
A: The effects of mitochondrial K-ATP channel opener DZ on apoptosis (TUNEL staining) and apoptosis-related gene (caspase-3) expression in spinal cord after ischemia-reperfusion injury (magnification, ×1000). SCI, spinal cord ischemia. B: The effects of mitochondrial K-ATP channel opener DZ on apoptosis-related proteins (PARP and AIF) in spinal cord after ischemia-reperfusion injury (magnification, ×1000). C: TUNEL staining in rabbit spinal cord after ischemia (30 minutes) and reperfusion (60 minutes) injury in the absence or presence of mitochondrial K-ATP channel opener DZ. D: Immunohistochemical staining of caspase-3, AIF, and PARP in rabbit spinal cord after ischemia (30 minutes) and reperfusion (60 minutes) injury in the absence or presence of mitochondrial K-ATP channel opener DZ.
Discussion
The processes by which ischemia-reperfusion injury lead to cell survival, necrosis, or apoptosis are numerous and dependent on the organ or tissue studied. However, it is likely that there are a limited number of initiating events that, when set in motion, result in the activation of ever-increasing numbers of cellular components designed to sustain the cell (anti-apoptotic), cause orderly cell demise (pro-apoptotic), or cause cell necrosis. In this report, we have shown that ROS are generated after reperfusion by mitochondria in the rabbit spinal cord subjected to ischemia. We have also shown that DZ provided before ischemia dramatically reduces the output of ROS and the many noxious downstream effects we measured, including neurological failure. These findings strengthen the case that ROS generation by damaged mitochondria initiate the intrinsic apoptotic pathway under the conditions of these experiments.
DZ is a mitochondrial membrane K-ATP channel opener that has been shown in multiple models, both in vivo and in vitro, to attenuate the response to ischemia-reperfusion injury. The mechanism is thought to be similar to that produced by ischemic preconditioning. DZ has been reported to exert this effect by sustaining the mitochondrial membrane potential, reducing calcium influx,34,35 and preventing opening of the mitochondrial permeability transition pore36,37 and release of cytochrome c.38,39 However, it has been reported that mitochondrial K-ATP channel openers function by regulation of mitochondrial volume; Garlid and colleagues40 have shown that the decreases in mitochondrial membrane potential and Ca uptake seen with DZ can be observed in K-free medium. We also observed important morphological differences by electron microscopy. After 30 minutes of ischemia and 1 hour of reperfusion, there was swelling, membrane breakage, and lack of definition of the cristae of the mitochondria in the untreated lumbar spinal cords. Mitochondrial architecture was well preserved in animals treated with DZ (Figure 2). Similar changes in structure have been correlated to physiological function in rat lung cells subject to ischemia and reperfusion in vitro.41 To our knowledge, this is the first report that correlates mitochondrial structure and function in an in vivo model of neurological injury.
Mitochondria are thought to be the main source of intracellular ROS during reoxygenation.30,41,42 Although ROS production in excess leads to cellular injury,30 it also signals cellular processes required in ischemic preconditioning.43,44 Forbes et al45 found that activation of mitochondrial K-ATP channels by DZ actually increased ROS production in a mild ischemic challenge in rat hearts and that addition of the antioxidant N-acetylcysteine blocked the effects of DZ. In addition, ROS can themselves activate mitochondrial K-ATP channels.46 Thus, ROS production resulting from small ischemic challenges protects cells. Our study involving a major ischemic challenge resulting in paraplegia clearly shows a high accumulation of ROS in the mitochondrial fraction. DZ pretreatment caused a decrease in intrinsic ROS production that was associated with improved mitochondrial morphology, cell survival, and function. This implies that a severe ischemic challenge results in significant derangement of mitochondrial oxidative phosphorylation on reperfusion that overpowers existing ROS scavengers and results in oxidative injury. By mechanisms not yet understood, DZ stabilizes mitochondria sufficiently to preserve cellular function. Vanden Hoek et al47 have also reported quantitative relationships using a mitochondrial K-ATP channel inhibitor in a rabbit myocardium preparation.
The concept that mitochondrial ROS production is paramount in reperfusion injury is supported by the finding of oxidative DNA injury, as estimated from determinations of 8-oxoG, the most stable and harmful adduct of oxidative damage (Figure 6).48,49 Blocking the production of excess ROS by DZ lessened oxidative DNA damage. Somewhat paradoxically, there was much greater activity of the DNA repair enzyme OGG-l in the spinal cords protected by DZ. We interpret this finding to mean that the injury in untreated cords was so extensive that transcription of DNA repair enzymes failed (Figure 7). When the injury was attenuated by DZ, DNA repair activity was evident.
There was also ample evidence of apoptosis in our model. The TUNEL reaction was positive with time, and immunohistochemical staining for the caspase-dependent (caspase-3) and -independent (AIF) apoptotic pathways was positive. At the time points we studied, there was minimal necrosis by light microscopy. Caspase-3 is the end effector of apoptosis that is activated by the apoptosome complex (consisting of Apaf-1 and caspase-9), which in turn is activated by cytochrome c release by the mitochondria.50–52 Akao et al20 have similarly shown that DZ maintained cardiomyocyte integrity in response to exogenous oxidative stress by preventing mitochondrial membrane depolarization with associated decreases in apoptosis-inducing events like cytochrome c translocation, caspase-3 activation, and PARP-1 cleavage in cultured rat myocytes. PARP-1 is a nuclear enzyme that responds to DNA single-strand breaks and that facilitates DNA repair and mediates cell death in many experimental models, including ROS-induced injury.53 Yu et al54 have shown that PARP-1 activation is required for translocation of AIF from the mitochondria to the nucleus and that AIF is necessary for PARP-dependent cell death initiated by hydrogen peroxide in fibroblasts, as well as by glutamate excitotoxicity in cortical neurons. AIF is normally present in the mitochondrial intermembrane space and, like cytochrome c, is released in response to mitochondrial injury. However, unlike cytochrome c, it causes apoptosis by a manner independent of the apoptosome complex.55
There are clearly other important participants in programed cell death that we have not evaluated. However, from what we have measured and, most importantly, from the marked physiological effects observed, we can conclude that the generation of superoxide and hydrogen peroxide in the mitochondria damaged by ischemia and re-exposed to oxygen leads to oxidative damage and the elaboration of multiple compounds culminating in apoptosis/necrosis in the lumbar spinal cord. The importance of ROS derived from mitochondria after ischemia-reperfusion injury is emphasized by our finding that DZ in dosages used clinically eliminated the excessive production of ROS and associated output of pro-apoptotic compounds, thus sustaining neurological function after an ischemic challenge that would otherwise uniformly cause paraplegia.
Footnotes
Address reprint requests to Chiming Wei, M.D., Ph.D., 600 N. Wolfe St., Blalock 1206, Baltimore, MD 21205. E-mail: cmwei@jhmi.edu.
Supported in part by the National Institutes of Health (grant no. HL61299 to C.W.), the Maryland Department of Health and Mental Hygiene (to C.W.), the Johns Hopkins Fund for Medical Discovery (to C.W.), and the Johns Hopkins Institutional Research grant (to C.W.).
G.R. and D.G. contributed equally to this work.
References
- Acher CW, Wynn MM, Hoch JR, Popic P, Archibald J, Turnipseed WD. Combined use of cerebral spinal fluid drainage and naloxone reduces the risk of paraplegia in thoracoabdominal aneurysm repair. J Vasc Surg. 1994;19:236–246. doi: 10.1016/s0741-5214(94)70099-0. [DOI] [PubMed] [Google Scholar]
- Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236:471–479. doi: 10.1097/00000658-200210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambria RP, Davison JK, Carter C, Brewster DC, Chang YC, Clark KA, Atamian S. Epidural cooling for spinal cord protection during thoracoabdominal aneurysm repair: a five-year experience. J Vasc Surg. 2000;31:1093–1101. doi: 10.1067/mva.2000.106492. [DOI] [PubMed] [Google Scholar]
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thor Surg. 1999;67:1931–1934. doi: 10.1016/s0003-4975(99)00390-2. [DOI] [PubMed] [Google Scholar]
- de Haan P. Pharmacologic adjuncts to protect the spinal cord during transient ischemia. Semin Vasc Surg. 2000;13:264–271. [PubMed] [Google Scholar]
- Jacobs MJ, Meylaerts SA, de Haan P, de Mol BA, Kalkman CJ. Strategies to prevent neurologic deficit based on motor-evoked potentials in type I and II thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1999;29:48–57. doi: 10.1016/s0741-5214(99)70349-6. [DOI] [PubMed] [Google Scholar]
- Kouchoukos NT, Masetti P, Rokkas CK, Murphy SF. Hypothermic cardiopulmonary bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 2002;74:S1885–S1887. doi: 10.1016/s0003-4975(02)04153-x. [DOI] [PubMed] [Google Scholar]
- Safi HJ, Bartoli S, Hess KR, Shenaq SS, Viets JR, Butt GR, Sheinbaum R, Doerr HK, Maulsby R, Rivera VM. Neurologic deficit in patients at high risk with thoracoabdominal aortic aneurysms: the role of cerebral spinal fluid drainage and distal aortic perfusion. J Vasc Surg. 1994;20:434–444. doi: 10.1016/0741-5214(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Safi HJ, Miller CC, III, Carr C, Iliopoulos DC, Dorsay DA, Baldwin JC. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1998;27:58–66. doi: 10.1016/s0741-5214(98)70292-7. [DOI] [PubMed] [Google Scholar]
- Safi HJ, Campbell MP, Miller CC, III, Iliopoulos DC, Khoynezhad A, Letsou GV, Asimacopoulos PJ. Cerebral spinal fluid drainage and distal aortic perfusion decrease the incidence of neurological deficit: the results of 343 descending and thoracoabdominal aortic aneurysm repairs. Eur J Vasc Endovasc Surg. 1997;14:118–124. doi: 10.1016/s1078-5884(97)80208-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Kristian T, Siesjo BK. Calcium-related damage in ischemia. Life Sci. 1996;59:357–367. doi: 10.1016/0024-3205(96)00314-1. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels: possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sato T, Seharaseyon J, Szewczyk A, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: viable candidate effectors of ischemic preconditioning. Ann NY Acad Sci. 1999;874:27–37. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Marban E. The role of mitochondrial K(ATP) channels in cardioprotection. Basic Res Cardiol. 2000;95:285–289. doi: 10.1007/s003950070047. [DOI] [PubMed] [Google Scholar]
- Akao M, Ohler A, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Yonemochi H, Sato T, Saikawa T. Diazoxide triggers cardioprotection against apoptosis induced by oxidative stress. Am J Physiol Heart Circ Physiol. 2003;284:H2235–H2241. doi: 10.1152/ajpheart.01073.2002. [DOI] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Takashi E, Wang Y, Ashraf M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- Teshima Y, Akao M, Li RA, Chong TH, Baumgartner WA, Johnston MV, Marban E. Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke. 2003;34:1796–1802. doi: 10.1161/01.STR.0000077017.60947.AE. [DOI] [PubMed] [Google Scholar]
- Caparrelli DJ, Cattaneo SM, Bethea BT, Shake JG, Eberhart C, Blue ME, Marban E, Johnston MV, Baumgartner WA, Gott VL. Pharmacological preconditioning ameliorates neurological injury in a model of spinal cord ischemia. Ann Thorac Surg. 2002;74:838–844. doi: 10.1016/s0003-4975(02)03716-5. [DOI] [PubMed] [Google Scholar]
- Lin RX, Roseborough G, Dong YF, Williams GM, Wei CM. DNA damage and repair system in spinal cord ischemia. J Vasc Surg. 2003;37:847–858. doi: 10.1067/mva.2003.150. [DOI] [PubMed] [Google Scholar]
- Sims NR. Selective impairment of respiration in mitochondria isolated from brain subregions following transient forebrain ischemia in the rat. J Neurochem. 1991;56:1836–1844. doi: 10.1111/j.1471-4159.1991.tb03438.x. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Kraimer JM, Graeber GM. Effects of flunarizine on neurological recovery and spinal-cord blood-flow in experimental spinal-cord ischemia in rabbits. Stroke. 1993;24:1547–1553. doi: 10.1161/01.str.24.10.1547. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Zhu H, Li YB, Lin HZ, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion: resolved and unresolved issues. Circulation. 1989;80:1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- Wei C, Jiang SW, Lust JA, Daly RC, McGregor CGA. Genetic expression of endothelial nitric oxide synthase in human atrial myocardium. Mayo Clin Proc. 1996;71:346–350. doi: 10.4065/71.4.346. [DOI] [PubMed] [Google Scholar]
- Lu AL. Repair of A/G and A/8-oxoG mismatches by MutY adenine DNA glycosylase. DNA Repair Protocols. 2000;152:3–16. doi: 10.1385/1-59259-068-3:3. [DOI] [PubMed] [Google Scholar]
- Hazra TK, Izumi T, Maidt L, Floyd RA, Mitra S. The presence of two distinct 8-oxoguanine repair enzymes in human cells: their potential complementary roles in preventing mutation. Nucleic Acids Res. 1998;26:5116–5122. doi: 10.1093/nar/26.22.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol. 1999;519:347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Nishigaki N, Hayashi H. Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation. 2002;105:2666–2671. doi: 10.1161/01.cir.0000016831.41648.04. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang XD. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu XS, Kim CN, Yang J, Jemmerson R, Wang XD. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- Ueda M, Kosaka S, Hirata T, Fukuse T, Suzuki Y, Hitomi S, Wada H. Mitochondrial injuries in rat lungs preserved for 17 h: an ultrastructural study. Eur Surg Res. 1999;31:162–172. doi: 10.1159/000008635. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Mitochondria and xanthine oxidase both generate reactive oxygen species in isolated perfused rat liver after hypoxic injury. Biochem Biophys Res Commun. 1989;160:140–147. doi: 10.1016/0006-291x(89)91632-x. [DOI] [PubMed] [Google Scholar]
- Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Chen YF, Zhang DX, Yi FX, Qi J, Andrade-Gordon P, de Garavilla L, Li PL, Zou AP. Urotensin II is a nitric oxide-dependent vasodilator and natriuretic peptide in the rat kidney. Am J Physiol Renal Physiol. 2003;285:F792–F798. doi: 10.1152/ajprenal.00342.2002. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-]T and A-]C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging-8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CYJ, Sasaki T, Elia AJ, Cheng HYM, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]