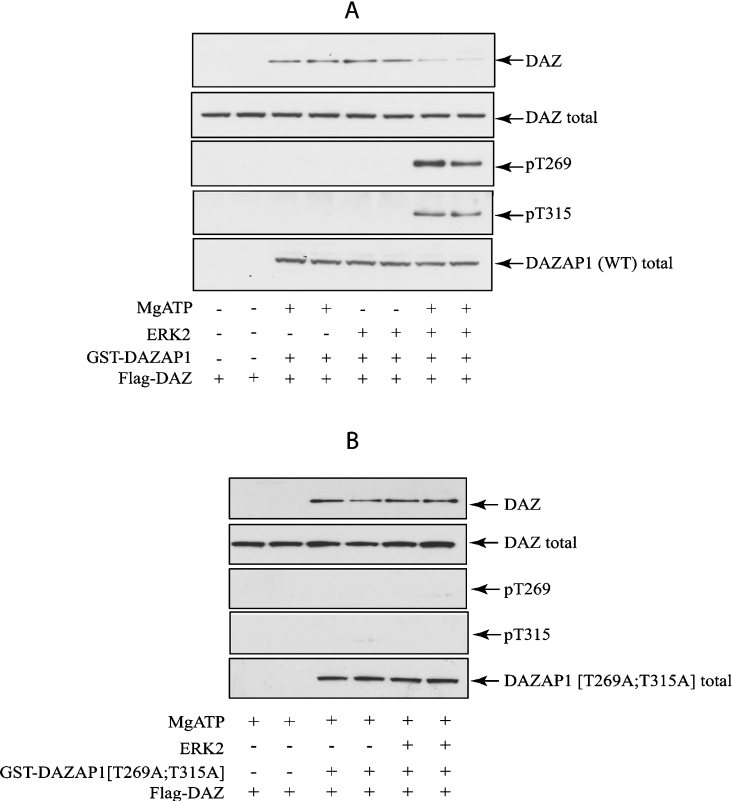
Figure 6. Phosphorylation of full-length DAZAP1 by ERK2 impairs its binding to DAZ.
HEK-293 cells were transfected with 1 μg of DNA encoding FLAG–DAZ and with 0.5 μg of DNA encoding GST–DAZAP1(WT) (A) or a non-phosphorylatable form GST–DAZAP1(T269A/T315A) (B). After 36 h, the cells were lysed and the extract (1 mg of protein) was incubated for 2 h at 4 °C with glutathione–Sepharose (20 μl). The beads were washed, and phosphorylated with ERK2 in the presence (+) or absence (−) of MgATP, before being washed again and denatured in SDS. Protein released from the beads was subjected to SDS/PAGE, transferred on to nitrocellulose membranes and immunoblotted. The GST–DAZAP1 in the extract (DAZAP1 total) was revealed with an anti-GST-antibody (DAZAP1) and FLAG–DAZ with an anti-FLAG antibody (DAZ). The membranes were also immunoblotted with the phospho-specific antibody that recognises DAZAP1 phosphorylated at Thr315 (pT315) and with the phospho-specific antibody that not only recognizes c-Jun phosphorylated at Thr91, but also DAZAP1 phosphorylated at Thr269 (pT269).