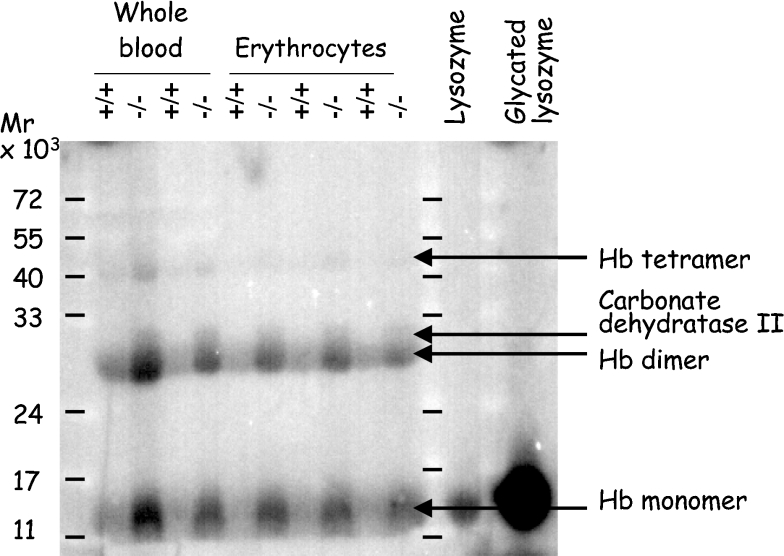
Figure 3. Phosphorylation of glycated residues from proteins in erythrocyte extracts from wild-type and Fn3k−/− mice.
Whole blood extracts and erythrocyte lysates from Fn3k+/+ and Fn3k−/− mice (100 μg per lane) or control lysozyme or glycated (25 μg per lane) were separated by SDS/PAGE, the proteins were transferred on to a nitrocellulose membrane and phosphorylated with recombinant E. coli fructoselysine 6-kinase and [γ-32P]ATP. The membrane was overloaded to enhance the signal of the faintest bands. As a result of this, the phosphorylation of the lower band, corresponding to haemoglobin monomers, is underestimated compared with the other bands.