Abstract
Evidence suggests that cyclooxygenase-2 (COX-2) increases tumorigenic potential by promoting resistance to apoptosis. Because B chronic lymphoid leukemia (B-CLL) cells exhibit a defective apoptotic response, we analyzed CD19+ B lymphocytes purified from the peripheral blood of B-CLL patients. Microarray analysis showed a variable (up to 38-fold) increase in the steady-state mRNA levels of COX-2 in B-CLL lymphocytes compared with normal CD19+ B lymphocytes. The up-regulation of COX-2 in B-CLL cells was confirmed by reverse transcriptase-polymerase chain reaction and Western blot analyses. Moreover, immunohistochemical analysis of B-CLL bone marrow infiltrates confirmed clear expression of COX-2 in leukemic cells. Ex vivo treatment with the COX-2 inhibitor NS-398 significantly decreased the survival of leukemic cells by increasing the rate of spontaneous apoptosis in 13 of 16 B-CLL samples examined, but it did not affect the survival of normal lymphocytes. Pretreatment with NS-398 significantly potentiated the cytotoxicity induced by chlorambucil in 8 of 16 B-CLL samples examined. Moreover, although recombinant tumor necrosis factor-related apoptosis inducing ligand (TRAIL)/Apo2L showed little cytotoxic effect in most B-CLL samples examined, pretreatment with NS-398 sensitized 8 of 16 B-CLL samples to TRAIL-induced apoptosis. Taken together, our data indicate that COX-2 overexpression likely represents an additional mechanism of resistance to apoptosis in B-CLL and that pharmacological suppression of COX-2 might enhance chemotherapy-mediated apoptosis.
Chronic lymphocytic leukemia (CLL) is a highly heterogeneous disease with one-third of patients never requiring treatment, whereas in other patients, the disease progresses at a variable rate.1,2 CLL represents a quintessential example of human malignancies that are caused primarily by defects in apoptosis.3,4 Defects in apoptotic pathways contribute to chemoresistance, rendering tumor cells less sensitive to the cytotoxic actions of currently available anticancer drugs.
Cyclooxygenase (COX)-1 and -2, also known as prostaglandin H synthases (PTGSs), catalyze one of the rate-limiting steps in the prostanoids biosynthesis.5,6 COX isoenzymes, which are encoded by two different genes, possess the same oxygenase and peroxidase activities and catalyze the formation of prostaglandin H2 from arachidonic acid. Despite the remarkable structural and functional homologies, COX-1 and COX-2 have been shown to preferentially couple with different isoforms of prostagladin synthases and subserve distinct functions even within the same cell.5 In particular, it has been clearly established that the COX-2 (or PTGS2) gene behaves like an immediate-early gene, being rapidly induced in response to mitogenic or inflammatory stimuli.5 The premise that COX-2 is involved in growth and progression of several types of solid cancers is strongly supported by both epidemiological and animal studies.7–10 Evidence suggests that the increase in tumorigenic potential by COX-2 overexpression is associated with resistance to apoptosis.11 Selective inhibitors of COX-2 have been shown to induce apoptosis in a variety of cancer cells, including those of colon, stomach, prostate, and breast.6,12–15 Although the role of COX-2 in lymphoid carcinogenesis is poorly defined, high levels of prostaglandins have been found in patients with lymphoma, and elevated levels of COX-2 protein have been detected in lymphoma cell lines.16,17
Microarray technology has been previously used to profile gene expression in B-CLL and to subcharacterize patients with heterogeneous clinical outcome.18–23 Because it has been clearly shown that B-CLL cells are defective in their apoptotic response, in searching for new potential therapeutic targets, we have analyzed by cDNA microarray the expression profile of a set of stress- and toxicity-associated genes, including COX-2/PTGS2, in B-CLL samples in comparison with normal B lymphocytes. Because COX-2 was identified as one of the up-regulated genes, we have further characterized the expression of COX-2 protein in B-CLL and its role on cell viability. Moreover, to investigate the therapeutic potential of a strategy based on COX-2 inhibition, viability of B-CLL cells was analyzed in response to NS-398, a selective pharmacological inhibitor of COX-2 activity, used alone or in association with chemotherapeutic agents or recombinant tumor necrosis factor-related apoptosis inducing ligand (TRAIL).
Materials and Methods
Patients and Cell Purification
Peripheral blood (PB) samples were collected from 10 healthy human blood donors and 16 B-CLL patients after informed consent, in agreement with institutional guidelines. The diagnosis of B-CLL was made by PB morphology and immunophenotyping. Patients were staged according to the Rai system (Table 1). Two independent blood collections were obtained from each B-CLL patient within a clinical follow-up time of 6 to 18 months. During this period, patients were clinically stable, with a lymphocyte doubling time of >12 months, free from infectious complications, and with no evidence of disease progression. Moreover, none of the patients received cytoreductive chemotherapy both before entering this study and during the follow-up period.
Table 1.
Clinical Characteristics of the B-CLL Patients
| Patient | Sex/Age | Months since diagnosis | WBC* | Stage† | Doubling time‡ |
|---|---|---|---|---|---|
| 1 | F/74 | 171 | 29.7 | 1 | 12 |
| 2 | M/65 | 20 | 25.6 | 0 | 20 |
| 3 | M/73 | 83 | 42.8 | 4 | 20 |
| 4 | F/60 | 59 | 47.6 | 0 | 29 |
| 5 | F/84 | 16 | 28.8 | 0 | 30 |
| 6 | M/71 | 27 | 18.6 | 1 | 33 |
| 7 | M/75 | 107 | 42.8 | 1 | 36 |
| 8 | M/53 | 8 | 12.1 | 0 | 45 |
| 9 | M/61 | 36 | 46.9 | 1 | 46 |
| 10 | M/61 | 34 | 46.3 | 1 | 46 |
| 11 | M/73 | 64 | 35.8 | 0 | 47 |
| 12 | M/63 | 40 | 25.9 | 1 | 49 |
| 13 | F/65 | 50 | 31.5 | 0 | 60 |
| 14 | M/63 | 64 | 24.6 | 0 | 96 |
| 15 | M/69 | 181 | 47.4 | 0 | 125 |
| 16 | M/58 | 158 | 27.7 | 2 | 130 |
×103 cells/ml.
Rai classification.
Months.
PB mononuclear cells (PBMCs) were separated by gradient centrifugation with lymphocyte cell separation medium (Cedarlane Laboratories, Hornby, Ontario, Canada). T lymphocytes and monocytes were depleted from normal PBMCs and, in some cases, from B-CLL with immunomagnetic microbeads (MACS microbeads; Miltenyi Biotech, Auburn, CA), resulting in purity of >90% CD19+ B cells, as assessed by flow cytometry. After purification, CD19+ B lymphocytes were resuspended at a cell density of 1 × 106 cells/ml in RPMI supplemented with 10% fetal calf serum (Gibco-BRL, Grand Island, NY), 2 mmol/L l-glutamine, and 40 mg/ml gentamicin sulfate in the absence of exogenous cytokines.
Flow Cytometric Analysis of Cell Surface Antigens
Surface expression of CD19 and CD5 was evaluated by direct staining with fluorescein isothiocyanate (FITC)-CD19 (Becton Dickinson Biosciences, San Jose, CA) and PE-CD5 (Immunotech, Marseille, France) monoclonal antibodies (mAbs). Surface expression of TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4 was evaluated by staining with primary mAbs (all from Alexis Biochemical, Lausen, Switzerland) followed by PE-conjugated anti-mouse secondary Ab (Immunotech). Nonspecific fluorescence was assessed using isotype-matched Abs. Flow cytometry analyses were performed by FACScan (Becton Dickinson).
cDNA Microarray and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from purified normal or B-CLL CD19+ cells by using the Qiagen RNeasy mini kit (Qiagen, Hilden, Germany) according to the supplier’s instructions. Three micrograms of total RNA were transcribed into cDNA using GEArray AmpoLabeling-LPR kit (Superarray Bioscience Corporation, Frederick, MD). An in vitro linear polymerase reaction was then performed to generate biotinylated cRNA. Labeled cDNA was hybridized with a customized cDNA microarray containing a panel of genes associated to stress and toxicity response (GEArray HS-603; SuperArray Bioscience Corporation). Hybridization was revealed by alkaline phosphatase-conjugated streptavidin, using a chemiluminescent detection kit (Superarray Bioscience Corporation). Signal intensity was measured for each microarray, the minimal intensity was used for background subtraction, and the values were normalized to the median signal value for each array. Expression levels were compared between the leukemic samples and normal CD19+ B lymphocytes, and then data were filtered for the genes whose expression level increased by at least twofold, that is, filtering the ratio for values ≥2.0.
To validate the accuracy of the genes selected on the microarray, the same RNA samples used for microarray hybridization were analyzed by semiquantitative RT-PCR. In particular, COX-2 mRNA amplification was performed using the following primers: forward, 5′-TCCTGGCGCTCAGCCATACAG-3′; reverse, 5′-GTAGCCATAGCTAGC-ATTGTA-3′.
Western Blotting Analysis
Western blot was performed on approximately 5 to 10 × 106 cells/sample. To obtain cell lysates, cell suspensions were mixed with a lysing buffer containing 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS and protease inhibitors (Protease inhibitor cocktail P8340; Sigma Chemical, St. Louis, MO). Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of proteins for each sample were migrated in 10% polyacrylamide gel electrophoresis and blotted onto nitrocellulose filters. Blotted filters were blocked for 60 minutes in a 3% suspension of dried skimmed milk in phosphate-buffered saline and incubated overnight at 4°C with Abs anti-COX-2 (Cayman Chemicals, Ann Arbor, MI), caspase-3, poly (ADP-ribose) polymerase (PARP), and tubulin (Santa Cruz Biotechnology, Santa Cruz, CA). Filters were washed and further incubated for 1 hour at room temperature with 1:1000 dilution of peroxidase-conjugated anti-mouse IgG (Sigma). Specific reactions were revealed with the ECL Western blotting detection reagent (Amersham Corp., Arlington Heights, IL).
Bone Marrow Specimens and Immunohistochemical Analysis
Needle biopsies taken from bone marrow of three B-CLL patients at Rai stage 2 were used for immunohistochemical analysis. Sections were processed as previously described.24 Briefly, antigen retrieval was performed by microwaving (three cycles lasting 5 minutes each at 900W) in 1 mmol of ethylenediamine tetraacetic acid (pH 8.0), and a three-layer alkaline phosphatase-anti alkaline phosphatase (APAAP) technique was applied for antigen detection. For COX-2 immunohistochemistry, the anti-COX-2 mAb (Cayman Chemicals; 1:20 dilution) was used. The specificity of the staining was ensured by using isotype-matched irrelevant antibody, as a substitute for primary antibody, and the immuno-alkaline phosphatase was adopted to avoid any disturbance from the endogenous peroxidase activity. Slides were analyzed blinded by two independent people.
Culture Treatments
NS-398, a relative specific pharmacological inhibitor of COX-2 isoenzyme (Biomol, Plymouth Meeting, PA) was dissolved in ethanol, and stock solution was stored at −20°C for no more than 3 months. NS-398 was used at the final concentrations of 12.5 to 200 μmol/L. DuP697, an unrelated pharmacological inhibitor of COX-2 (Sigma Chemical) was dissolved in ethanol and used at the final concentration of 100 μmol/L. Chlorambucil and Fludarabine (F-ara-A) (Sigma) were added at the final concentration of 10 μmol/L. Recombinant histidine 6-tagged TRAIL(114–281), produced in bacteria and purified by chromatography, as described previously,25 was added at the final concentration of 1 μg/ml.
PGE2 levels were measured in the supernatants of cell cultures using the prostaglandin E2 EIA kit-monoclonal (Cayman Chemicals), following manufacturer’s instructions. Cell viability was assessed by trypan blue dye exclusion, and the degree of apoptosis was assessed by annexin V-FITC/propidium iodide double staining (Trevigen Inc., Gaithersburg, MD) followed by flow cytometric analysis and in parallel by analyzing cell lysates for PARP cleavage in Western blot.
Statistical Analysis
Box plots were used to show the median, minimum, maximum values and 25th to 75th percentiles of the RNA levels for each group of data. Correlation coefficients were calculated by Spearman’s method. Results were evaluated by using analysis of variance with subsequent comparisons by Student’s t-test and with the Mann-Whitney rank-sum test. Statistical significance was defined as P < 0.05.
Results
COX-2 Is Up-Regulated in B-CLL in Comparison with Normal B Cells
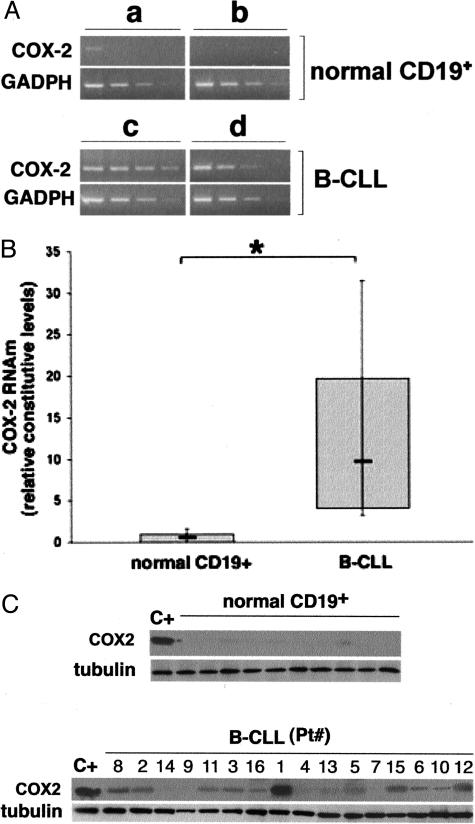
All B-CLL patients who entered this study (Table 1) were previously untreated, and we did not pre-select any subgroup of B-CLL patients to represent the heterogeneity of this disease course. RNA was extracted from eight freshly purified B-CLL PBMCs containing an excess of B leukemic cells (>85% of CD19+/CD5+ B cells) and from three normal B lymphocyte samples (>90% CD19+) and analyzed by cDNA microarray for a set of stress and toxicity associated genes (GEArray HS-603; SuperArray Bioscience Corporation), which included PTGS2/COX-2. The concept of peripheral blood B cells being appropriate controls for B-CLL cells in microarray analysis has been recently validated by different authors.18,22 Genes up-regulated in all B-CLL included IL-1β, IL-8, BCL3, and NOS2A in agreement with previous studies.23,26–28 For the purpose of this study, however, it is particularly remarkable that PTGS2/COX-2 was also consistently up-regulated, at variable levels (mean fold of increase compared CD19+ B lymphocytes, 12.4; range, 2 to 33.3) in all of the B-CLL samples examined. Validation of the COX-2 microarray results was performed by RT-PCR on the same samples analyzed by microarray approach (Figure 1A).
Figure 1.
COX-2 expression in B-CLL and in normal CD19+ B lymphocytes. A: Microarray results were validated by RT-PCR on serial scalar dilutions of the RNA templates. Amplification of the glyceraldehyde-3-phosphate dehydrogenase (GADPH) housekeeping gene was used to confirm comparability of the samples. Representative results obtained from two normal CD19+ B lymphocyte and two B-CLL samples are shown. B: COX-2 mRNA levels were measured in CD19+ B lymphocytes purified from PBMCs of 10 different healthy donors and in 16 B-CLL patients after normalization to the level of GADPH mRNA. Each sample was determined in duplicate. Horizontal bars are median; top and bottom edges of box are 75th and 25th percentiles; lines extending from box are 10th and 90th percentiles. *P < 0.01. C: COX-2 protein was analyzed by Western blot in lysates obtained from normal CD19+ cells purified from PBMCs of 10 different healthy donors, and from samples of 16 B-CLL patients (Pt.). Comparable loading of protein in each lane was confirmed by staining with the antibody to tubulin (C+, positive control).
Furthermore, quantitative analysis of the steady-state mRNA levels of COX-2 was extended to a total of 16 B-CLL samples and 10 normal B cell samples (Figure 1B). Overall, a significant (P < 0.01) increase of COX-2 mRNA levels was demonstrated in the B-CLL samples examined over normal B lymphocytes (Figure 1B). Because COX-2 is irreversibly inactivated following catalysis, it is assumed that COX activity is determined by the amount of the enzyme protein and is regulated exclusively at the levels of transcription and translation. Consistent with this hypothesis, the results of RT-PCR were further confirmed by analyzing COX-2 protein at Western blot. In fact, as shown in Figure 1C, whereas COX-2 protein was virtually undetectable in normal B cells, it was detected, at variable levels, in the majority of B-CLL samples examined. The relative expression levels of COX-2 protein, determined by densitometry analysis, significantly correlated (r = 0.81, P < 0.01) with the steady-state mRNA levels.
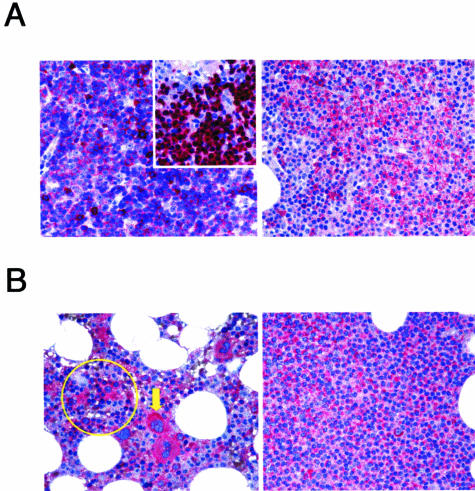
In additional experiments, COX-2 expression was examined in biopsies obtained from B-CLL patients with leukemic bone marrow infiltrates. As shown in a representative sample (Figure 2A), the leukemic bone marrow infiltrate displayed a nodular pattern mainly consisting of small lymphocytes and pro-lymphocytes. These cells exhibited the characteristic B-CLL immunophenotype, ie, positivity for CD79a, CD23, and CD5. When the bone marrow biopsies were immunostained for COX-2 antigen, COX-2 expression was observed in normal erythroblasts and megakaryocytes (Figure 2B, left panel), as expected on the basis of previous studies of our and other groups of investigators.29–31 More importantly, clear expression of COX-2 protein was detected also in the leukemic infiltrate (Figure 2B, right panel).
Figure 2.
COX-2 expression in B-CLL bone marrow biopsies. Biopsies from bone marrow of B-CLL patients were analyzed by immunohistochemistry. A: Positivity for CD5 (left panel), CD79a (inset), and CD23 (right panel) of a nodular infiltrate in the bone marrow. B: COX-2 expression in normal erythroblasts (circle) and megakaryocytes (arrowhead) in the normal bone marrow (left panel) as well as in the nodular infiltrates (right panel). A representative sample is shown. Original magnification, ×400.
The Selective COX-2 Inhibitor NS-398 Induces Cytotoxicity in Most B-CLL Samples but Not in Normal Purified CD19+ B Lymphocytes and PBMCs
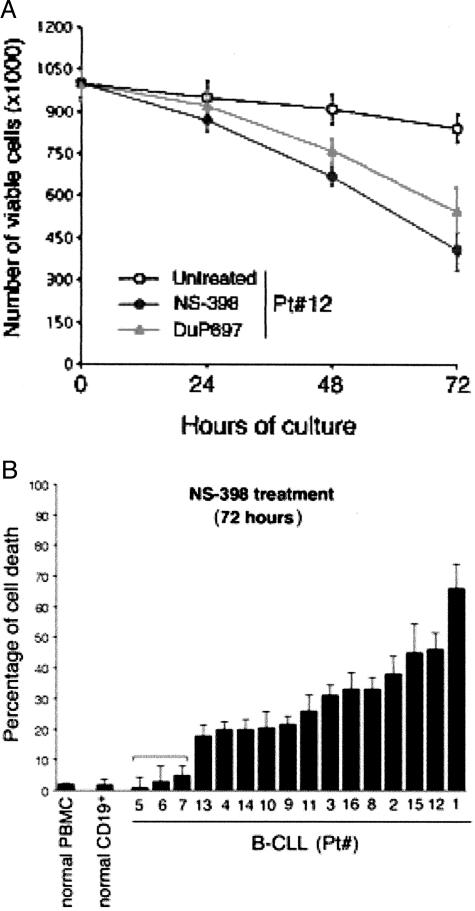
In the next group of experiments, B-CLL cells were cultured ex vivo for 72 hours in the absence or presence of the pharmacological inhibitor of COX-2, NS-398. This time frame was chosen taking into account that in the absence of exogenous cytokines, which are known to prolong the in vitro lifespan of B-CLL cells,26–28 leukemic cells did not proliferate ex vivo and the number of viable cells remained relatively constant, never dropping below 80% of the total cell number seeded at time 0 (Figure 3A). The effect of NS-398 on cell viability was monitored every 24 hours, as exemplified in Figure 3A for one B-CLL sample. In some B-CLL cultures (n = 3), a structurally unrelated COX-2 inhibitor (DuP697; 100 μmol/L) was also used, to rule out possible bystander effects of NS-398 on B-CLL cell viability. As shown in Figure 3A, also DuP697 induced a significant decrease in the number of viable cells, comparable with that observed with NS-398.
Figure 3.
Effect of NS-398 on ex vivo cultured B-CLL cells, normal CD19+ B lymphocytes and normal PBMCs. Primary normal CD19+ B lymphocytes, PBMCs, and leukemic cells isolated from B-CLL patients were cultured in the absence or presence of COX-2 inhibitors. A: After 24, 48, and 72 hours of treatment, cell viability was monitored by scoring the total number of viable cells. Shown are results from one representative B-CLL patient. Data are means ± SD of results from two independent experiments each performed in triplicate. B: Cytotoxicity measured after 72 hours of NS-398 treatment is reported for normal PBMCs (n = 8), for CD19+ B lymphocytes (n = 4), and for each B-CLL patient analyzed. Data are expressed as percentage of control untreated cultures and are means ± SD of three experiments, each performed in triplicate. In samples 5, 6, and 7, NS-398 treatment did not significantly affect viability.
The susceptibility of each B-CLL sample to the ex vivo NS-398 treatment is reported for the end point (72 hours) in Figure 3B. In 13 of 16 B-CLL patients, NS-398 induced a significant (P < 0.01, cut-off >20%) decline in viability with respect to untreated control cultures (32 ± 16% of cytotoxicity, means ± SD of 13 cases), whereas no significant variations were observed in terms of viable cell number in only 3 of 16 B-CLL samples, as well as in normal purified CD19+ B lymphocytes (n = 4) and in total PBMCs (n = 8) (Figure 3B). There were no apparent correlations between sensitivity to NS-398 and clinical characteristics of the B-CLL patients. Moreover, analysis of the samples examined by cDNA microarray did not reveal any correlation between sensitivity to NS-398 and the steady-state mRNA levels of Bcl-2 (r = 0.48, P > 0.05).
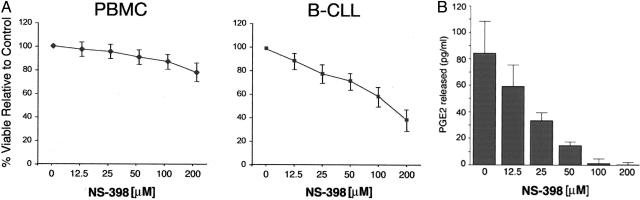
Cell viability was next measured in either B-CLL or primary normal PBMCs incubated with various concentrations of NS-398 for 72 hours (Figure 4A). NS-398 induced a dose-dependent cytotoxic effect when added to B-CLL leukemic cells, whereas normal PBMCs were refractory to NS-398 cytotoxicity even at the highest drug concentrations (200 μmol/L) (Figure 4A). It should be noted that a 24-hour treatment with the concentration of NS-398 used in this and in the following set of experiments (100 μmol/L) was able to completely block the production and the release in culture of prostaglandins (PGE2) in five different B-CLL samples examined (Figure 4B).
Figure 4.
Dose-dependent cytotoxicity and inhibition of PGE2 release by NS-398 in B-CLL samples. Normal PBMCs and leukemic cells isolated from B-CLL patients were cultured in the absence or presence of NS-398. A: Mean dose response curves for B-CLL cells isolated from patients (n = 10) and for normal PBMCs isolated from healthy blood donors (n = 6) cultured with increasing doses of NS-398 for 72 hours. Percentage of viable cells relative to control (vehicle alone) is expressed on the y axis. B: PGE2 release was measured in the supernatant of B-CLL cells (n = 5) cultured with increasing doses of NS-398. Results are expressed as means ± SD.
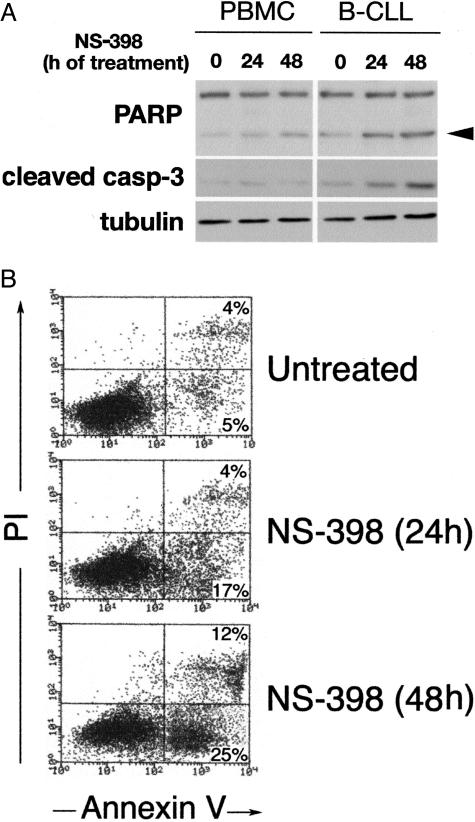
Concurrently, we sought to elucidate whether the cytotoxicity of NS-398 toward B-CLL cells was caused by apoptosis. For this purpose, B-CLL cell lysates were examined for the levels of PARP, a downstream target of activated caspase-3 that is typically cleaved in the setting of caspase-mediated apoptosis. In NS-398-treated B-CLL cells, Western blot analysis revealed the increase of the 85-kd cleaved product of PARP (Figure 5A). Moreover, exposure to NS-398 resulted in a significant (P < 0.05) increase in the percentage of Annexin V-positive cells compared with untreated cultures (Figure 5B). Taken together, these findings suggest that COX-2 overexpression plays a role in counteracting the apoptotic pathway in B-CLL cells and demonstrate that B-CLL cells are more susceptible to NS-398-induced cell death compared with normal purified CD19+ B lymphocytes and PBMCs.
Figure 5.
NS-398 induces apoptotic cell death in B-CLL cells. Normal PBMCs and leukemic cells isolated from B-CLL patients were cultured in the absence or presence of NS-398. A: Protein lysates from B-CLL cultures treated as indicated were analyzed in Western blot for PARP and caspase-3 cleavage. Arrowhead indicates the 85-kd cleaved product of PARP. Representative results obtained from PBMCs and B-CLL cells are shown. B: Apoptosis was monitored in flow cytometry by annexin V-FITC binding and propidium iodide staining. Representative results obtained from B-CLL cultures are shown.
NS-398 Potentiates the Cytotoxicity of Chemotherapeutic Agents and TRAIL in a Subset of B-CLL Samples
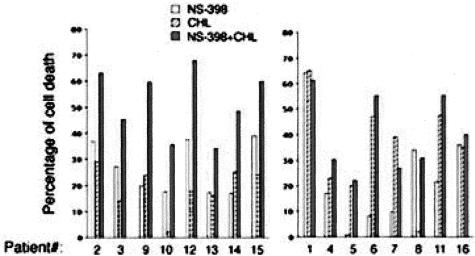
We next investigated whether NS-398, besides showing a significant cytotoxic activity on B-CLL samples when used alone, was able to modulate the susceptibility of B-CLL cells to chemotherapeutic agents. When B-CLL cells were exposed in culture to NS-398 for 24 hours before adding chlorambucil for additional 48 hours, a significant (P < 0.05) increase of cytotoxicity with respect to either chlorambucil or NS-398, used as single agents, was observed in 8 of the 16 patients examined (Figure 6). It is noteworthy that all B-CLL samples examined, except cells from patient 8, were susceptible to chlorambucil-mediated apoptosis. Interestingly, this sample was susceptible to NS-398-mediated apoptosis, suggesting that NS-398 might be pharmacologically active also in chlorambucil-resistant B-CLL cells. Similar findings were obtained when fludarabine (F-ara) was used instead of chlorambucil (data not shown).
Figure 6.
Cytotoxic effect of NS-398 in combination with chlorambucil on B-CLL cells. B-CLL samples were pre-incubated with NS-398 for 24 hours before exposure to chlorambucil (CHL) for an additional 48 hours. The cytotoxic effect of the treatment with the single agents (NS-398 and CHL alone) and with the combination is reported for each patient analyzed. Data are expressed as percentage of control untreated cultures and are means of the results from two independent experiments, each performed in duplicate. SDs were comprised within 10%. The patients in which NS-398+CHL treatment induced greater apoptosis levels versus the treatment with the single agents are grouped in the left graphic.
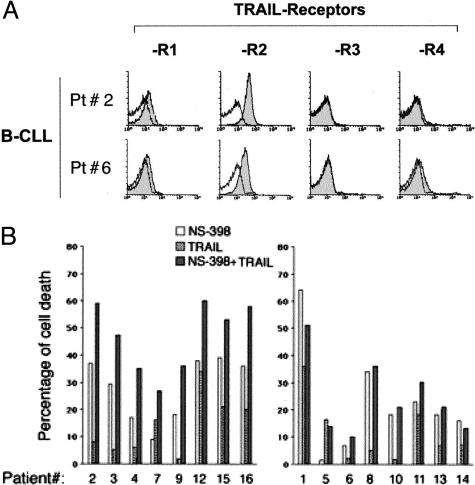
In parallel, the ability of NS-398 to sensitize to cell death was also examined in cultures treated with recombinant TRAIL. Despite the fact that all B-CLL samples examined showed significant surface levels of the death receptor TRAIL-R2 (Figure 7A), recombinant TRAIL alone induced a modest cytotoxic response when added alone to B-CLL cells (Figure 7B). These findings were not unexpected on the basis of previous data of different groups of investigators including ourselves.32,33 Nevertheless, when B-CLL cells were pretreated for 24 hours with NS-398 before adding TRAIL for additional 48 hours, a significant (P < 0.05) increase of cytotoxicity with respect to both recombinant TRAIL and NS-398, used as single agents, was observed in 8 of 16 B-CLL samples (Figure 7B).
Figure 7.
Cytotoxic effect of NS-398 in combination with TRAIL on B-CLL cells. A: Surface expression of TRAIL receptors (TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4) was analyzed by flow cytometry in B-CLL PBMCs. Shadowed histograms represent cells stained with mAbs for the indicated antigens, whereas unshadowed histograms represent the background fluorescence obtained from the staining of the same cultures with isotype-matched control mAbs. Representative phenotypes are shown. B: B-CLL samples were pre-incubated with NS-398 for 24 hours before exposure to TRAIL for additional 48 hours. The effect of the treatment with the single agents (NS-398 and TRAIL alone) and with the combination is reported for each patient analyzed. Data are expressed as percentage of control untreated cultures and are means of the results from two independent experiments, each performed in duplicate. SDs were comprised within 10%. The patients in which NS-398+TRAIL treatment induced greater apoptosis levels versus the treatment with the single agents are grouped in the left graphic.
Discussion
Gene expression profiling has been used in previous studies to compare the molecular differences and similarities between leukemic B-CLL cells and healthy cells as well as to compare the molecular signatures of subsets of malignant cells based on their biology or clinical outcome.18–23 B-CLL samples have been shown to possess a gene expression profile related to resting and memory B cells, not in keeping with the hypothesis that CLL cells are derived from CD5+ B cells.18 These studies have increased our understanding of CLL pathogenesis, classification, and risk stratification.
By comparing B-CLL cells with healthy peripheral blood B cells, we have identified COX-2 as a potential therapeutic target for the treatment of B-CLL. In fact, the steady-state mRNA levels of COX-2 were variably up-regulated in B-CLL samples in comparison with normal B cells. In this respect, it is noteworthy that several mechanisms have been proposed by which COX-2 overexpression inhibits apoptosis, including increased Bcl-2 expression,15 and up-regulation of the anti-apoptotic kinase Akt.34 Consistently, we were able to document a variable up-regulation of the mRNA steady-state levels of Bcl-2 (from 2 to 6.5 fold) in a subset of B-CLL samples in our microarray analysis (data not shown). Moreover, other authors have shown that the levels of Akt activity are pathologically elevated in B-CLL cells in comparison with normal lymphocytes.35,36
The relevance of COX-2 up-regulation in protecting B-CLL cells from apoptosis was underscored by the fact that pharmacological inhibition of COX-2 activity by NS-398 induced a significant increase of cytotoxicity in most B-CLL samples examined and showed an additive or synergistic cytotoxic effect when added in combination with either chemotherapeutic agents or TRAIL. In a previous paper, Bellosillo et al37 suggested a COX-2-independent cytotoxic effect of aspirin in a small cohort of B-CLL patients studied; and in five of seven patients examined, NS-398 (used at the same concentrations used in this study) failed to induce significant apoptosis. Although we do not have a ready explanation for these discrepancies, Bellosillo et al did not specify the percentage of leukemic cells in the PBMC samples examined. In this respect, it is important to underline that in our study only patients with a number of leukemic cells higher than 85% were enrolled. Because the mean apoptosis induced by NS-398 in B-CLL samples was 32 ± 16% over the untreated cultures, a low purity of starting cell population could easily mask the effect of NS-398.
Our current findings that COX-2 is overexpressed in primary leukemic B-CLL cells expand previous findings, which have documented that COX-2 is overexpressed in solid tumors and represent a potential target for therapy.38–40 As with other malignancies, the accumulation of genetic abnormalities is required for malignant transformation of human lymphocytes. Whether COX-2 might contribute to malignant progression of B-CLL is uncertain. COX-2 is a bifunctional enzyme with both oxidation and peroxidation activities. There is recent evidence that COX-2 peroxidation can lead to the production of mutagens, such as malondialdehyde.41 Although we cannot exclude that the increased COX-2 expression in B-CLL may represent an epiphenomenon representative of activation mechanisms, it is also possible that COX-2 overexpression plays a role in the pathogenesis of B-CLL, like in solid tumors.38 Because COX-2 is an immediate-early response gene, induced by a variety of cytokines, the observed up-regulation of COX-2 in B-CLL cells might be secondary to the release of cytokines in an autocrine fashion. Consistent with this hypothesis, our microarray analysis revealed a marked elevation of IL-8 and even more pronounced of IL-1β mRNA, in keeping with previous findings of other authors.42,43
In addition to genetic evidence, numerous pharmacological studies suggest that COX-2 is a bona fide therapeutic target in solid tumors.38–40 Treatment with selective inhibitors of COX-2 reduced the formation of tongue, esophageal, intestinal, breast, skin, lung, and bladder tumors in experimental animals.38–40 Whether pharmacological inhibitors of COX-2 suppress carcinogenesis exclusively by inhibiting COX-2 is not certain. For example, high concentrations of selective COX-2 inhibitors suppress the growth of cells in culture that do not express COX-2, and new celecoxib derivatives without COX-2 inhibitory activity have been recently proposed for the treatment of B-CLL.44,45 It is therefore possible that both COX-2-dependent and COX-2-independent anticarcinogenic effects account for the cytotoxicity of NS-398 also in B-CLL, as previously demonstrated in colon cancer.46
Although our study suggests that COX-2 inhibitors might be also effective in B-CLL, it seems unrealistic to expect that selective COX-2 inhibitors will be useful as monotherapy in treating B-CLL. In all likelihood, selective COX-2 inhibitors will need to be given in conjunction with either standard anticancer therapy or with innovative therapy, in the effort to re-establish a normal apoptotic process as a therapeutic approach in B-CLL.47 Taken together, our results suggest that COX-2 warrants investigation as a molecular target for the prevention and treatment of B-CLL.
Acknowledgments
We thank Dr. Milena Piccioli for her technical support and Dr. Fortunato Morabito for providing clinical data.
Footnotes
Address reprint requests to Paola Secchiero, Ph.D., Department of Morphology and Embryology, Human Anatomy Section, University of Ferrara, Via Fossato di Mortara 66, 44100 Ferrara, Italy. E-mail: secchier@mail.umbi.umd.edu.
Supported by Fondo per L’Incentivazione Della Ricerca di Base (FIRB) (to P.S. and G.Z.), by Associazione Italiana per la Ricerca sul Cancro (AIRC) (to G.Z.), and CAN2005 Project—“Comitato dei Sostenitori” (to P.S.).
References
- Dighiero G, Binet JL. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343:1799–1801. doi: 10.1056/NEJM200012143432410. [DOI] [PubMed] [Google Scholar]
- Pangalis GA, Vassilakopoulos TP, Dimopoulou MN, Siakantaris MP, Kontopidou FN, Angelopoulou MK. B-cronic lymphocytic leukemia: practical aspects. Hematol Oncol. 2002;20:103–146. doi: 10.1002/hon.696. [DOI] [PubMed] [Google Scholar]
- Masdehors P, Merle-Beral H, Maloum K, Omura S, Magdelenat H, Delic J. Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood. 2000;96:269–274. [PubMed] [Google Scholar]
- Reed JC, Kitada S, Kim Y, Byrd J. Modulating apoptosis pathways in low-grade B-cell malignancies using biological response modifiers. Semin Oncol. 2002;29:10–24. doi: 10.1053/sonc.2002.30155. [DOI] [PubMed] [Google Scholar]
- Ueno N, Murakami M, Tanioka T, Fujimori K, Tanabe T, Urade Y, Kudo I. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. J Biol Chem. 2001;276:34918–34927. doi: 10.1074/jbc.M100429200. [DOI] [PubMed] [Google Scholar]
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- Kutchera W, Jones DA, Matsunami N, Groden J, McIntyre TM, Zimmerman GA, White RL, Prescott SM. Prostaglandin H synthase 2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Dubois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Hara A, Yoshimi N, Niwa M, Ino N, Mori H. Apoptosis induced by NS-398, a selective cyclooxygenase-2 inhibitor, in human colorectal cancer cell lines. Jpn J Cancer Res. 1997;88:600–604. doi: 10.1111/j.1349-7006.1997.tb00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson BA, Longo WE, Panesar N, Mazuski JE, Kaminski DL. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res. 1999;81:101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;274:G1061–G1067. doi: 10.1152/ajpgi.1998.274.6.G1061. [DOI] [PubMed] [Google Scholar]
- Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- Sebahoun G, Maraninchi D, Carcassonne Y. Increased prostaglandin E production in malignant lymphomas. Acta Haematol. 1985;74:132–136. doi: 10.1159/000206188. [DOI] [PubMed] [Google Scholar]
- Wun T, McKnight H, Tuscano JM. Increased cyclooxygenase-2 (COX-2): a potential role in the pathogenesis of lymphoma. Leuk Res. 2004;28:179–190. doi: 10.1016/s0145-2126(03)00183-8. [DOI] [PubMed] [Google Scholar]
- Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat L, Magdelenat H, Merle-Beral H, Masdehors P, Potocki de, Montalk G, Davi F, Kruhoffer M, Sabatier L, Orntoft TF, Delic J. The resistance of B-CLL cells to DNA damage-induced apoptosis defined by DNA microarrays. Blood. 2003;101:4598–4606. doi: 10.1182/blood-2002-06-1743. [DOI] [PubMed] [Google Scholar]
- Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100:9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gricks CS, Zahrieh D, Zauls AJ, Gorgun G, Drandi D, Mauerer K, Neuberg D, Gribben JG. Differential regulation of gene expression following CD40 activation of leukemic compared to healthy B cells. Blood. 2004;104:4002–4009. doi: 10.1182/blood-2004-02-0494. [DOI] [PubMed] [Google Scholar]
- Stratowa C, Loffler G, Lichter P, Stilgenbauer S, Haberl P, Schweifer N, Dohoner H, Wilgenbus KK. cDNA microarray gene expression analysis of B-cell chronic lymphocytic leukemia proposes potential new prognostic markers involved in lymphocyte trafficking. Int J Cancer. 2001;91:474–480. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1078>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pileri SA, Sabattini E, Agostinelli C, Bodega L, Rossi M, Zinzani PL, Marafioti T. Histopathology of B-cell chronic lymphocytic leukemia. Hematol Oncol Clin N Am. 2004;18:807–826. doi: 10.1016/j.hoc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Gonelli A, Celeghini C, Mirandola P, Guidotti L, Visani G, Capitani S, Zauli G. Activation of the nitric oxide synthase pathway represents a key component of the tumor necrosis factor-related apoptosis-inducing ligand-mediated cytotoxicity on hematological malignancies. Blood. 2001;98:2220–2228. doi: 10.1182/blood.v98.7.2220. [DOI] [PubMed] [Google Scholar]
- Francia di Celle P, Mariani S, Riera L, Stacchini A, Reato G, Foa R. Interleukin-8 induces the accumulation of B-cell chronic lymphocytic leukemia cells by prolonging survival in an autocrine fashion. Blood. 1996;87:4382–4389. [PubMed] [Google Scholar]
- Hulkkonen J, Vilpo J, Vilpo L, Koski T, Hurme M. Interleukin-1beta, interleukin-1 receptor antagonist and interleukin-6 plasma levels and cytokine gene polymorphisms in chronic lymphocytic leukemia: correlation with prognostic parameters. Haematologica. 2000;85:600–606. [PubMed] [Google Scholar]
- Levesque MC, Misukonis MA, O’Loughlin CW, Chen Y, Beasley BE, Wilson DL, Adams DJ, Silber R, Weinberg JB. IL-4 and interferon gamma regulate expression of inducible nitric oxide synthase in chronic lymphocytic leukemia cells. Leukemia. 2003;17:442–450. doi: 10.1038/sj.leu.2402783. [DOI] [PubMed] [Google Scholar]
- Rocca B, Secchiero P, Ciabattoni G, Ranelletti FO, Catani L, Guidotti L, Melloni E, Maggiano N, Zauli G, Patrono C. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc Natl Acad Sci USA. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca B, Secchiero P, Celeghini C, Ranelletti FO, Ciabattoni G, Maggiano N, Habib A, Ricerca BM, Barbarotto E, Patrono C, Zauli G. Modulation of the expression and activity of cyclooxygenases in normal and accelerated erythropoiesis. Exp Hematol. 2004;32:925–934. doi: 10.1016/j.exphem.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Sato T, Fujita H, Morita I. Constitutive expression and involvement of cyclooxygenase-2 in human megakaryocytopoiesis. Arterioscler Thromb Vasc Biol. 2004;24:607–612. doi: 10.1161/01.ATV.0000117181.68309.10. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, Cohen GM. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21:6809–6818. doi: 10.1038/sj.onc.1205853. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Tiribelli M, Barbarotto E, Celeghini C, Michelutti A, Masolini P, Fanin R, Zauli G. Aberrant expression of TRAIL in B chronic lymphocytic leukemia (B-CLL) cells. J Cell Physiol. 2005;205:246–252. doi: 10.1002/jcp.20392. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cδ. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–2976. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- Bellosillo B, Pique M, Barragan M, Castano E, Villamor N, Colomer D, Montserrat E, Pons G, Gil J. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406–1414. [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Plastaras JP, Guengerich FP, Nebert DW, Marnett LJ. Xenobiotic-metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J Biol Chem. 2000;275:11784–11790. doi: 10.1074/jbc.275.16.11784. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (Abeta 1–42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005;159:66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Farrajota K, Cheng S, Martel-Pelletier J, Afif H, Pelletier JP, Li X, Ranger P, Fahmi H. Inhibition of interleukin-1beta-induced cyclooxygenase 2 expression in human synovial fibroblasts by 15-deoxy-Delta12,14-prostaglandin J2 through a histone deacetylase-independent mechanism. Arthritis Rheum. 2005;52:94–104. doi: 10.1002/art.20714. [DOI] [PubMed] [Google Scholar]
- Waskewich C, Blumenthal RD, Li H, Stein R, Goldenberg DM, Burton J. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2-negative hematopoietic and epithelial cell lines. Cancer Res. 2002;62:2029–2033. [PubMed] [Google Scholar]
- Johnson AJ, Smith LL, Zhu J, Heerema NA, Jefferson S, Mone A, Grever M, Chen CS, Byrd JC. A novel celecoxib derivative, OSU03012, induces cytotoxicity in primary CLL cells and transformed B-cell lymphoma via a caspase and Bcl-2 independent mechanism. Blood. 2005;105:2504–2509. doi: 10.1182/blood-2004-05-1957. [DOI] [PubMed] [Google Scholar]
- Maier TJ, Schilling K, Schmidt R, Geisslinger G, Grosch S. Cyclooxygenase-2 (COX-2)-dependent and -independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. Biochem Pharmacol. 2004;67:1469–1478. doi: 10.1016/j.bcp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Kolb JP, Kern C, Quiney C, Roman V, Billard C. Re-establishment of a normal apoptotic process as a therapeutic approach in B-CLL. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:261–286. doi: 10.2174/1568006033481384. [DOI] [PubMed] [Google Scholar]