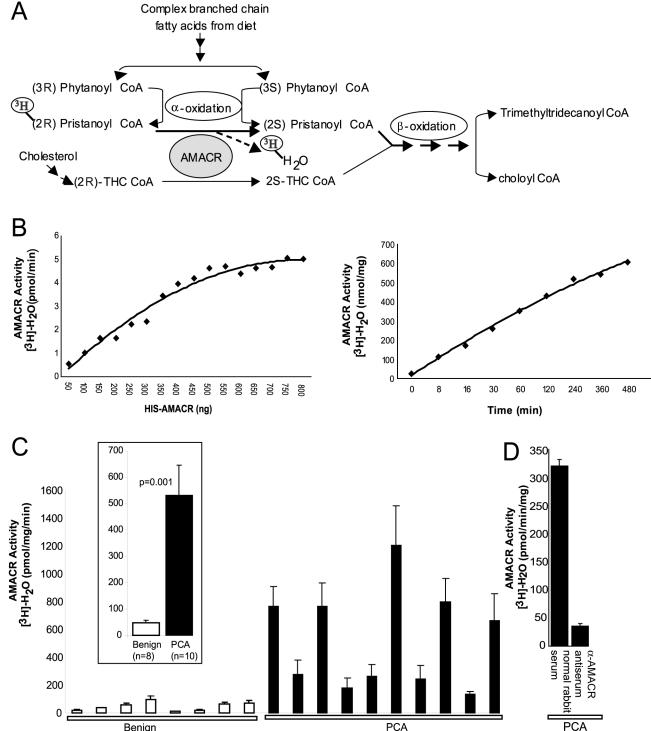
Figure 2.
AMACR enzymatic activity in prostate cancer. A: Schematic representation of the role of AMACR in facilitating β-oxidation of branched chain fatty acids by converting (R)-stereoisomer of pristanoyl-CoA and THC-CoA to their respective (S)-stereoisomers. The schematic also depicts the rationale of AMACR activity assay involving breakdown of [3H]-(2R)-pristanoyl CoA leading to release of [3H]-H2O. B: Standardization of the AMACR activity assay using recombinant AMACR. Enzymatic activity of AMΑCR expressed as the amount of [3H]-H2O generated per unit time, on incubation of 100 μmol/L substrate, [2-3H] pristanoyl-CoA, with increasing amounts of purified HIS-AMACR protein, for 30 minutes (left). Enzymatic activity of AMACR expressed as the amount of [3H]-H2O generated per mg of enzyme, on incubation of 100 μmol/L substrate, [2-3H] pristanoyl-CoA, with 250 ng of purified HIS-AMACR protein, throughout a time course (right). C: AMACR enzymatic activity in extracts from frozen tissue samples of grossly dissected benign or clinically localized prostate cancers (PCA). Tissue extracts were incubated with 100 μmol/L substrate, [2-3H] pristanoyl-CoA, expressed as the amount of [3H]-H2O generated per unit time, per unit total protein concentration. The values shown are mean ± SEM of three independent experiments. The inset represents a consolidated plot of the values for benign and PCA samples. D: Specific immunodepletion of AMACR enzymatic activity from prostate cancer extracts. The values shown are mean ± SEM of three independent assays.