Abstract
The cyclic AMP (cAMP)-signaling pathway regulates cell morphology and plays a crucial role during pathogenic development of the plant-pathogenic fungus Ustilago maydis. Strains lacking components of this signaling pathway, such as the Gα-subunit Gpa3 or the adenylyl cyclase Uac1, are nonpathogenic and grow filamentously. On the other hand, strains exhibiting an activated cAMP pathway due to a dominant-active allele of gpa3 display a glossy colony phenotype and are unable to proliferate in plant tumors. Here we present the identification of sql2 as a suppressor of the glossy colony phenotype of a gpa3Q206L strain. sql2 encodes a protein with similarity to CDC25-like guanine nucleotide exchange factors, which are known to act on Ras proteins. Overexpression of sql2 leads to filamentous growth that cannot be suppressed by exogenous cAMP, suggesting that Sql2 does not act upstream of Uac1. To gain more insight in signaling processes regulated by Sql2, we isolated two genes encoding Ras proteins. Expression of dominant active alleles of ras1 and ras2 showed that Ras2 induces filamentous growth while Ras1 does not affect cell morphology but elevates pheromone gene expression. These results indicate that Ras1 and Ras2 fulfill different functions in U. maydis. Moreover, observed similarities between the filaments induced by sql2 and ras2 suggest that Sql2 is an activator of Ras2. Interestingly, sql2 deletion mutants are affected in pathogenic development but not in mating, indicating a specific function of sql2 during pathogenesis.
Corn smut disease is caused by the fungal pathogen Ustilago maydis. The most prominent symptom of this disease is the formation of large plant tumors (18). The fungal mycelium proliferates within these tumors and finally differentiates into diploid spores (5). In U. maydis, pathogenic and sexual development are intimately connected. Fusion of two nonpathogenic haploid cells and subsequent development of the filamentous dikaryon are compulsory for pathogenesis (33). These processes are under the control of two mating-type loci termed a and b. Cell-cell recognition and fusion are controlled by the a locus carrying the pheromone (mfa) and receptor (pra) genes, while the switch to filamentous growth and all following developmental steps depend on the b locus (4, 11). The b locus codes for two unrelated homeodomain proteins (bE and bW), which form an active transcription factor only when they are derived from different alleles (27, 36). When haploid strains differ in their a and b mating-type alleles, they are termed compatible; i.e., they can fuse and undergo sexual and pathogenic development.
During the development of U. maydis, the transcription of genes in the a and b loci is coregulated; prior to fusion, pheromone secreted by haploid cells induces the transcription of the a and b genes in a compatible partner; after fusion, an autocrine pheromone stimulus triggers the expression of the active b heterodimer (64). The pheromone-induced transcription, as well as the basal transcription of the a and b genes, is mediated by the HMG domain transcription factor Prf1 (30). Prf1 is presumed to be activated by the Kpp2/Ubc3 MAP kinase module as well as the cAMP pathway, and both pathways are involved in the regulation of pheromone-responsive gene expression (40, 49). Interestingly, both signaling cascades also influence cell morphology, but they do so in opposing manners. While the mitogen-activated protein kinase (MAPK) module positively regulates filamentous growth, cAMP signaling triggers budding growth. The core components of the MAPK cascade (Kpp2/Ubc3, Fuz7, and Kpp4/Ubc4) (3, 6, 46, 49; P. Müller et al., submitted for publication), as well as of the cAMP cascade, have been characterized. An unknown signal initiates cAMP signaling by activating adenylyl cyclase Uac1, which produces the second messenger cAMP (7). Increasing concentrations of cAMP activate the cAMP-dependent protein kinase (PKA) composed of the regulatory subunit Ubc1 and the catalytic subunit Adr1 (23, 29). Strains disrupted in uac1 or adr1 grow filamentously, whereas strains exhibiting constitutively active PKA caused by the deletion of ubc1 show a cytokinesis defect termed multiple budding (7, 23, 29). Since all these mutants are nonpathogenic, regulated PKA activity appears to be crucial for pathogenic development.
In recent years, it has become evident that cAMP signaling plays a key role during development and pathogenesis in a variety of pathogenic fungi (22). Thus, detailed insight into the regulation of adenylyl cyclases is expected to improve a general understanding of the signaling mechanisms linking development and pathogenicity. In Saccharomyces cerevisiae, adenylyl cyclase Cyr1p is regulated by two small G-proteins, Ras1p and Ras2p (37, 61). In addition to Ras, the Gα-subunit Gpa2p plays the central roles in activating Cyr1p after the action of glucose stimuli (35, 50). In U. maydis, adenylyl cyclase appears to be positively regulated by the G-protein α-subunit Gpa3 (30). Strains expressing a dominant-active allele of gpa3, which codes for a α-subunit with strongly reduced GTPase activity, show elevated pheromone gene expression but exhibit an unaltered cell morphology (54). In addition, such strains are able to induce tumors in plants, but they do not proliferate inside the plant tissue (41). Another characteristic phenotype of gpa3Q206L strains is a glossy colony phenotype (41). Previously, we isolated suppressors of the glossy colony phenotype of gpa3Q206L strains. One of the identified genes was sql1, coding for a tetratricopeptide-repeat-type repressor related to Ssn6p of S. cerevisiae (44). Overexpression of sql1 triggered filamentous growth in wild-type cells, presumably by interfering with cAMP signaling on the transcriptional level (44). In this report we describe the characterization of a second gene, sql2, identified in the same screen. sql2 encodes a protein with similarities to guanine nucleotide exchange factors (GEFs) of the CDC25-family. Since Ras proteins are the main effectors of CDC25-like proteins, we have also cloned the genes ras1 and ras2 and have analyzed the phenotype of strains expressing dominant-active alleles of either ras1 or ras2.
MATERIALS AND METHODS
Strains and strain constructions.
For cloning purposes, the Escherichia coli K-12 derivative DH5α was used. U. maydis strains FB1 (a1b1), FB2 (a2b2), FBD11 (a1a2b1b2), FB1gpa3QL and FB2gpa3QL have been described previously (4, 54). FB1Pcrg1:fuz7DD has been described (Müller et al., submitted). Strains FB1Δsql2 and FB2Δsql2 were generated by transformation of the wild-type strains with plasmid pΔsql2 that had been digested with DraI. FB1POTEF:sql2, FB2POTEF:sql2, FB1gpa3QLPOTEF:sql2, and FB2gpa3QLPOTEF:sql2 were created by transforming the respective progenitor strains with plasmid pOTEF:sql2 that had been digested with DraI. In all cases, homologous integration was verified by Southern analysis. FB1Pcrg1:ras1Q67L was created by transformation of FB1 with pRU11ras1Q67L digested with SspI. Single homologous integration into the ip locus was verified by Southern analysis as described previously (44). FB2Δsql2[pNEBUC] and FB2Δsql2[pNEBUC-sql2] were generated by transformation of FB2Δsql2 with plasmids pNEBUC and pNEBUC-sql2, respectively, which had been digested with SspI prior to transformation. Single ectopic integration events were confirmed by Southern analysis.
To generate FB2ras2Q65L strains, we transformed FB2 with plasmid pRas2Q65Lcut with DraI. Among 48 transformants screened, we were unable to identify transformants showing homologous integration of the construct. Therefore, we continued to work with three independent transformants harboring single ectopic integrations at different sites in the genome.
Growth conditions for U. maydis.
U. maydis strains were grown as described previously (15). To assay the effects of cAMP on morphology, strains were grown in potato dextrose (PD) liquid medium and on charcoal-containing PD plates to which cAMP was added at the concentrations indicated in the descriptions of the respective experiments. For induction of the crg1 promoter, strains were grown in complete medium containing 1% glucose to an optical density at 600 nm of 0.8, washed twice in water, and resuspended in complete medium containing 1% arabinose. Hygromycin B was purchased from Roche, phleomycin was purchased from Cayla, and carboxin was purchased from Riedel de Haën. cAMP was added directly to the medium, which was filter sterilized before use. All other chemicals were of analytical grade and were obtained from Sigma or Merck.
The mating reaction was observed by cospotting strains onto charcoal-containing PD plates after incubation at 24°C for 48 h. Plant infections of the corn variety Early Golden Bantam were performed as described previously (49).
Isolation of sql2, ras1, and ras2.
To screen for suppressors of FB1gpa3QL the pCM54 library was used as described previously (44). Plasmid pSQL2 harboring an 8-kb fragment containing the entire open reading frame (ORF) of sql2 and approximately 1.7-kb 5′ as well as 3′ sequences was isolated. The entire insert was sequenced. With primer combinations ras1f (TACCATTGAGGACTCTTACC) plus ras1r (CGGCAGTATCCAACACATC) and ras2f (ACCATTGAAGACTCGTATCG) plus ras2r (CGCCGGTGTCCAACAGTAC), we amplified PCR fragments for ras1 and ras2, respectively. We used these fragments for hybridization to filters of a cosmid library (10). From the respective cosmids, we cloned a 3.5-kb EcoRI genomic fragment encompassing the ras1 gene as well as a 3.8-kb EcoRI fragment harboring the ras2 fragment into pTZ18R to obtain pRAS1E and pRAS2E, respectively. To detect the putative intron in ras1, we amplified the respective ORF with primers Ras1-5/2 (GATACAAAGTAACATCGACC) plus Ras1-3/1 (TCTAATGCTGGACAAGGTCG), with cDNA derived from FBD12 as template. The PCR-fragment was cloned into pCR2.1-TOPO and sequenced.
Plasmids and plasmid constructions.
Plasmids pTZ19R, pTZ18R (Pharmacia), pBS(−)SKII (Stratagene), and pCR2.1TOPO (Invitrogen) were used for cloning, subcloning, and sequencing. pΔsql is a pBS(−)SKII derivative in which three fragments were ligated into PstI and KpnI sites. The first 1-kb NsiI-SspI fragment (the 5′ part of sql2) and the second 1.8-kb HindIII-KpnI fragment (the 3′ part of sql2) were isolated from pSQL2. As the third fragment, we used a 2.8-kb SspI-HindIII fragment from pSLHyg(−) (15) containing the hygromycin resistance gene. To obtain pOTEF:sql2, we constructed pNEBUC-sql2 by cloning a 6.7-kb MscI-PflMI (blunt) fragment of pSQL2 encompassing the entire ORF of sql2 into the PmeI site of pNEBUC (G. Weinzierl et al., submitted for publication). The resulting plasmid, pNEBUC-sql2, was digested with BamHI and BglII and subsequently ligated with the otef promoter derived as a 0.8-kb BamHI-BamHI fragment from pCU4 (44), resulting in pNEBUC-OTEF:sql2. To insert the 5′ region of sql2 and the hygromycin resistance cassette, we performed a three-fragment ligation: the 5′ region of sql2 on a 1.1-kb BamHI-AgeI fragment from pΔsql2 and the hygromycin resistance cassette, isolated as a 2.9-kb XmaI-BamHI fragment from pNEBHyg(−)ΔEco (G. Weinzierl and R. Kahmann, unpublished data), were ligated into BamHI-cleaved pNEBUC-OTEF:sql2. From the resulting plasmid, we isolated a 6.2-kb EcoRI-NsiI fragment encompassing the 5′ region of sql2, the hygromycin resistance cassette, the otef promoter, and the ORF of sql2 up to bp +1183 and cloned it into EcoRI-PstI-digested pTZ19R. The resulting plasmid was termed pOTEF:sql2.
Plasmid pRas2Q65L contains the 5′ region, the ras2Q65L allele, a hygromycin resistance cassette, as well the as 3′ region of ras2. To obtain pRas2Q65L, we first used PCR to introduce a mutation into codon 65 (CAGGAG to CTCGAG), leading to an additional XhoI site. For this purpose, we performed two PCRs: for the first, we used primers Ras2-5/1 (AGAATTCCATATGAGTGGCAAAATGATG) and Ras2-3/3 (CTCGAGACCAGCCGTGTCAAG); for the second, we used primers Ras2-5/-1 (CTCGAGGAGTACACTGCG); and Ras2-3/2 (GATATCGCTTTCAAAGGATATTGC), introducing an EcoRV site at the stop codon. Then we amplified the 3′ region of ras2 by PCR with Ras2KO-5/1 (TCGATATCAAGCAAGCCTCAACAG) and RasKO-3/1 (TAGGGAAATCGCGGCCGC). These PCR products were cloned into pCR2.1TOPO to obtain pCRas2QL-I, pCRas2QL-II, and pCRas2-III, and the inserts were checked for correctness by sequencing. Then the 5′ fragment of ras2 was isolated as a 0.9-kb NspI-HindIII fragment from pRAS2E, the 5′ part of the ORF was obtained as a 177-bp HindIII-XhoI fragment from pCRas2QL-I, the 3′ part of the ORF was isolated as a 0.4-kb XhoI-EcoRV fragment from pCRas2QL-II, and, finally, the 3′ fragment of ras2 was isolated as a 0.5-kb EcoRV-EcoRI fragment from pCRas2QL-III. These four fragments were cloned into pTZ18R digested with EcoRI-SphI. The resulting plasmid was opened by digestion with EcoRV and ligated with a 2.5-kb NruI-DraI phleomycin resistance cassette derived from pSLBle(+) (Weinzierl et al., submitted), finally resulting in pras2Q65L.
Plasmid pRU11ras1Q67L was derived from pRU11, which is a plasmid for ip-locus integration. pRU11 harbors the sgfp gene under the control of the crg1 promoter and the nos-terminator (15). To obtain pRU11ras1Q67L, we replaced sgfp with the ras1Q67L allele. To this end, we performed PCRs to introduce the Q67L mutation that leads to an additional XhoI site. In the first PCR with Ras1-5′-NdeI (CATATGTCCAAAGCACAATTCTTG) and Ras1-Q65L (GACAGCTGTATTCCTCGAGACCAGCTGTATCC), we introduced an NdeI site at the ATG and an XhoI site at codon 67. The second PCR was performed with RasQLXhoI (CCGCTCGAGGAATACAGCTGTCAGAAGG) and Ras/3′NotI (GCGGCCGCTTAGAGAACGATACATTTCTGG), resulting in the amplified 3′ part of ras1. Both PCR products were cloned into pCR2.1TOPO and sequenced. To obtain pRU11ras1Q67L, these fragments were then isolated as 0.2-kb NdeI-XhoI and 0.6-kb XhoI-NotI fragments, respectively, and ligated with both 4.7-kb BglI-NdeI and 3.2-kb BglI-NotI fragments of pRU11 (15).
DNA and RNA procedures.
Standard molecular techniques were used (56). Transformation of U. maydis was performed as published previously (58). U. maydis DNA was isolated by the method described previously (32). RNA from liquid medium was prepared as published previously (40). The following probes were used for Northern analyses: for sql2, a 2.4-kb NcoI-DraI fragment derived from pNEBUC-sql2; for cbx, a 1.9-kb NotI-NotI fragment isolated from pNEBUC; a 675-bp EcoRV fragment spanning the mfa1 gene isolated from pUMa1 (64); and for frb34, a 1-kb EcoRI isolated from the respective pCR2.1-TOPO clone (15). A 5′-end-labeled oligonucleotide complementary to the U. maydis 18S rRNA (12) was hybridized as a loading control in Northern analyses. Radioactive labeling was performed with the NEBlot kit (New England Biolabs). A PhosphorImager (Storm 840; Molecular Dynamics) and the program IMAGEQUANT (Molecular Dynamics) were used for visualization and quantification of radioactive signals. Sequence information was obtained using an ABI 373 automated sequencer. Sequence analysis was performed with standard bioinformatics tools.
Light microscopy.
For microscopic observation, we used a Zeiss Axiophot microscope with differential interference contrast optics. The pictures were taken using a charge-coupled device camera (C4742-95; Hamamatsu, Herrsching, Germany). Image processing were done with Image Pro, Adobe Photoshop 6.0, and CanvasT 6.0 (Deneba Systems). For documentation, colonies were magnified with an Olympus binocular magnifier.
Nucleotide sequence Accession numbers.
The sequence data have been submitted to the GenBank database under accession numbers AY149915 for sql2, AY149916 for ras2, and AY149917 for ras1.
RESULTS
Isolation of sql2 as suppressor of the glossy colony phenotype of a gpa3Q206L strain.
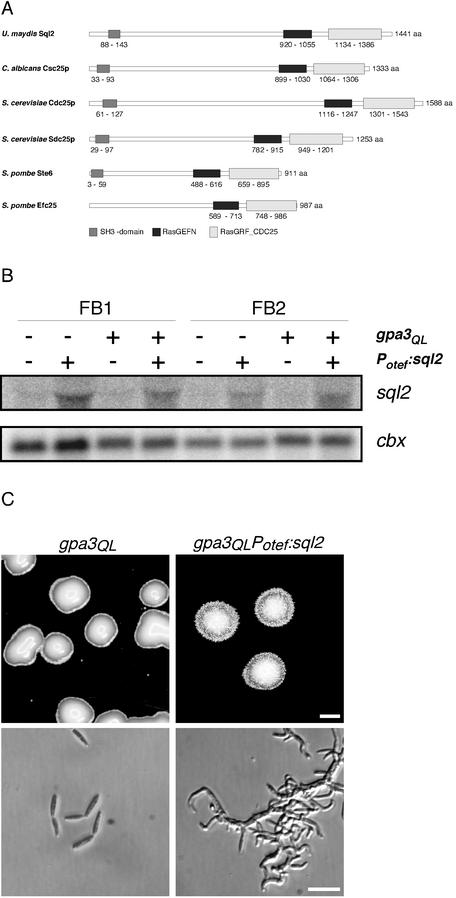
Plasmid pSQL2 was isolated by its ability to suppress the glossy colony phenotype of a gpa3Q206L strain. pSQL2 contained an 8-kb DNA fragment, and the suppressing activity was mapped to the insert (data not shown). Subsequent sequencing revealed a single ORF of 4,323 bp with no indication for introns. This gene was termed sql2 and encodes a putative protein of 1,441 amino acids. To confirm that sql2 is a multicopy suppressor of the glossy colony phenotype of a gpa3Q206L strain, we placed the gene under the control of the constitutively strong otef promoter (59) by replacing the endogenous promoter of sql2 in FB1gpa3QL (a1b1) and FB2gpa3QL (a2b2). In the resulting strains, FB1gpa3QLPOTEF:sql2 and FB2gpa3QLPOTEF:sql2, sql2 mRNA was approximately threefold more abundant than in the respective progenitor strains (Fig. 1B). In contrast to the glossy progenitor strain, colonies of these strains did not appear glossy on plates (Fig. 1C, upper panel, shown for FB1gpa3QLPOTEF:sql2). To analyze the cell morphology, we propagated FB1gpa3QLPOTEF:sql2 as well as FB1gpa3QL in liquid medium. While FB1gpa3QL cells were indistinguishable from the wild type, FB1gpa3QLPOTEF:sql2 formed aggregates of irregularly branched cells and the individual cells appeared elongated and curved (Fig. 1C, lower panel). This indicates that the suppressing effect of sql2 is associated with a dramatic change in cellular morphology.
FIG. 1.
(A) Domain structure of Sql2 and its homologues. Domains indicated below were identified using SMART and CDART (http://smart.embl-heidelberg.de/ and http://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi?cmd=rps). Domain annotations: SH3, pfam0001; RasGEFN, smart00229; and RasGRF_CDC25, IPR001895. Numbers represent the amino acid (aa) positions of each domain. Accession numbers: Sql2, AY149915; Csc25p, S30356; Cdc25p, CAA27259; Sdcp, P14771; Ste6, P26674; and Efc25, T40241. (B) Overexpression of sql2 with the otef promoter. Strains indicated at the top were incubated in PD liquid medium; 15 μg of total RNA was loaded per lane. The same filter was hybridized in succession with probes for sql2 and cbx as loading control. (C) Overexpression of sql2 suppresses the glossy colony appearance of a gpa3Q206L strain. FB1 derivatives indicated at the top were grown on PD-charcoal plates (upper panel) or in PD liquid medium (lower panel). Bars, 1 mm (upper panel) and 20 μm (lower panel).
Analysis of the deduced amino acid sequence of sql2 with SMART and CDART (43, 57) revealed that Sql2 contains a N-terminal SH3 domain (amino acids 88 to 143), known to be involved in protein-protein interactions, and indicated a RasGEFN domain (amino acids 920 to 1055) and a RasGRF_CDC25 domain (amino acids 1134 to 1386) (Fig. 1A) located in the C terminus. The last two domains are characteristic for proteins belonging to the family of GEFs (guanine nucleotide exchange factors). Such proteins promote the loss of bound GDP and the uptake of GTP in small G-proteins, leading to activation (9, 13). On the basis of sequence similarities, GEFs are classified into two families, the CDC24 family and the CDC25 family. While members of the CDC24 family act on Ras-like G-proteins such as Rho, CDC25 family members activate Ras proteins. Sql2 displayed no similarities to proteins of the CDC24 family but showed significant similarity to CDC25 family members over its entire length (Cdc25p of Candida albicans, 43%; Cdc25p and Sdc25p of S. cerevisiae, 40 and 37%, respectively; Ste6 and Efc25 of Schizosaccharomyces pombe, 36 and 37%, respectively [Fig. 1A]). Therefore, we conclude that Sql2 belongs to the CDC25 family of GEFs and might act on Ras proteins in U. maydis.
Deletion of sql2 does not affect mating but interferes with pathogenic development.
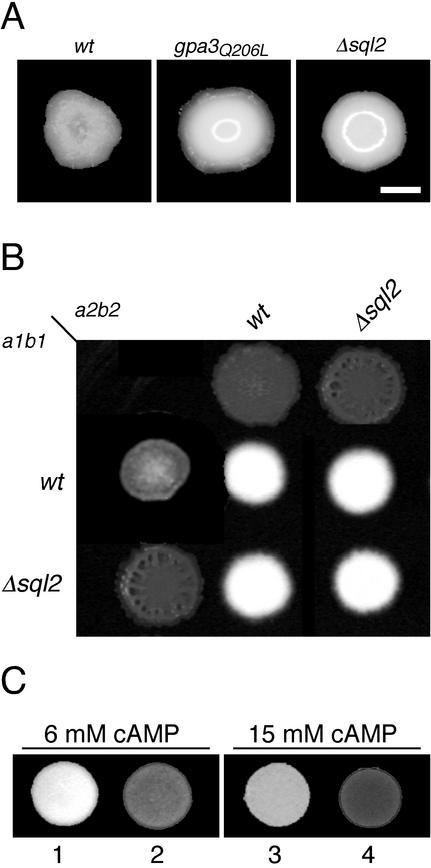
To analyze the function of sql2 in more detail, we constructed Δsql2 deletion strains. To this end, we deleted almost the whole coding region of sql2 (from positions +22 to +4276) in strains FB1 (a1b1) and FB2 (a2b2) by replacing this region with a hygromycin resistance cassette (see Materials and Methods). The resulting Δsql2 deletion strains were viable, showed no apparent growth defect, and displayed a wild-type budding pattern (not shown). Colonies of Δsql2 deletion mutants exhibited a glossy phenotype, which is similar to gpa3Q206L strains, while colonies of the wild-type strain appeared dull (Fig. 2A). It has been shown that the glossy colony phenotype of gpa3Q206L strains is correlated with production of an extracellular matrix and does not reflect a special growth mode (41).
FIG. 2.
(A) Deletion of sql2 leads to a glossy colony appearance. FB1 wild type (wt) and derivatives indicated at the top were grown on PD-charcoal plates. Bar, 1 mm. (B) Mating of sql2 deletion strains. Strains indicated at the top are FB2 (wt) and derivatives; strains listed on the left are FB1 (wt) and derivatives. Strains were spotted alone or in combinations on PD-charcoal plates and incubated at 24°C for 48 h. (C) Formation of dikaryotic filaments of sql2 deletion strains is sensitive to cAMP. Mixtures of FB1 and FB2 (1 and 3) and mixtures of FB1Δsql2 and FB2Δsql2 (2 and 4) were spotted on PD-charcoal plates with 6 mM cAMP (left panel) or 15 mM cAMP (right panel) and incubated at 24°C for 48 h.
The mating ability of Δsql2 mutants was tested in a plate mating assay, in which the fusion products, the dikaryotic hyphae, appear as white fuzzy filaments (Fig. 2B). Mixtures of compatible Δsql2 strains developed large numbers of dikayotic hyphae and in this respect resembled wild-type strains (Fig. 2B). This demonstrated that sql2 is not necessary for successful recognition, fusion, and filamentous growth.
Interestingly, in the presence of 6 mM cAMP, dikaryon formation of compatible Δsql2 strains was attenuated, while compatible wild-type strains still formed filaments (Fig. 2C). Moreover, we could hardly detect any dikaryotic filaments in mixtures of compatible Δsql2 strains when 15 mM cAMP was added to the plates, while under these conditions the wild-type strains showed only a slight reduction of dikaryon formation (Fig. 2B, right panel). It has been demonstrated that high levels of exogenous cAMP (25 mM) inhibit this dimorphic switch (29); however, dikaryon formation of Δsql2 strains is inhibited by much lower cAMP concentrations. Thus, Δsql2 strains may be more sensitive to exogenous cAMP, suggesting an influence of sql2 on the intracellular cAMP content.
To analyze whether sql2 plays a role during pathogenic development, we infected plants with mixtures of compatible Δsql2 mutants. Only 10 of 106 infected plants developed tumors (Table 1). This outcome suggests that sql2 plays an important role during pathogenesis, although it is dispensable for mating. All 10 tumors observed contained fungal spores (data not shown), indicating that lack of sql2 did not affect fungal development inside the plant tumor. Introduction of the wild-type sql2 gene into plasmid pNEBUC-sql2 but not of the empty vector into FB2Δsql2 restored pathogenicity (in mixtures with FB1Δsql2), demonstrating that the reduction in tumor development was due to deletion of sql2 (Table 1).
TABLE 1.
Plant infection assays
| Inoculum | No. of infected plants | No. of plants with tumors | % Tumor formation |
|---|---|---|---|
| FB1 (a1b1) × FB2 (a2b2) | 100 | 72 | 72 |
| FB1Δsql2 × FB2Δsql2 | 106 | 10 | 9 |
| FB1POTEF:sql2 × FB2POTEF:sql2 | 97 | 81 | 83 |
| FB1Δsql2 × FB2 | 16 | 10 | 83 |
| FB1Δsql2 × FB2Δsql2[pNEBUC] | 16 | 1 | 6 |
| FB1Δsql2 × FB2Δsql2[pNEBUC-sql2]#2 | 15 | 11 | 73 |
| FB1Δsql2 × FB2Δsql2[pNEBUC-sql2]#5 | 14 | 10 | 71 |
Overexpression of sql2 does not suppress the proliferation defect of gpa3Q206L strains.
Compatible strains harboring the gpa3Q206L allele are able to induce tumors in corn plants. However, such tumors remain hard and green, most probably due to the observed proliferation defect of gpa3Q206L strains (41). Consequently, we wondered whether overexpression of sql2 would also suppress the proliferation defect of gpa3Q206L strains in plant tumor tissue. For this purpose, we infected corn plants with mixtures of FB1gpa3QLPOTEF:sql2 and FB2gpa3QLPOTEF:sql2. Since it has been shown that the otef promoter is active in all developmental stages of the fungus, in the strains used sql2 should be overexpressed in planta (59). Plant tumors induced by these mixtures resembled tumors induced by compatible gpa3Q206L strains (data not shown), demonstrating that overexpression of sql2 does not suppress the proliferation defect of gpa3Q206L strains.
Overexpression of sql2 induces filamentous growth but does not affect pathogenic growth.
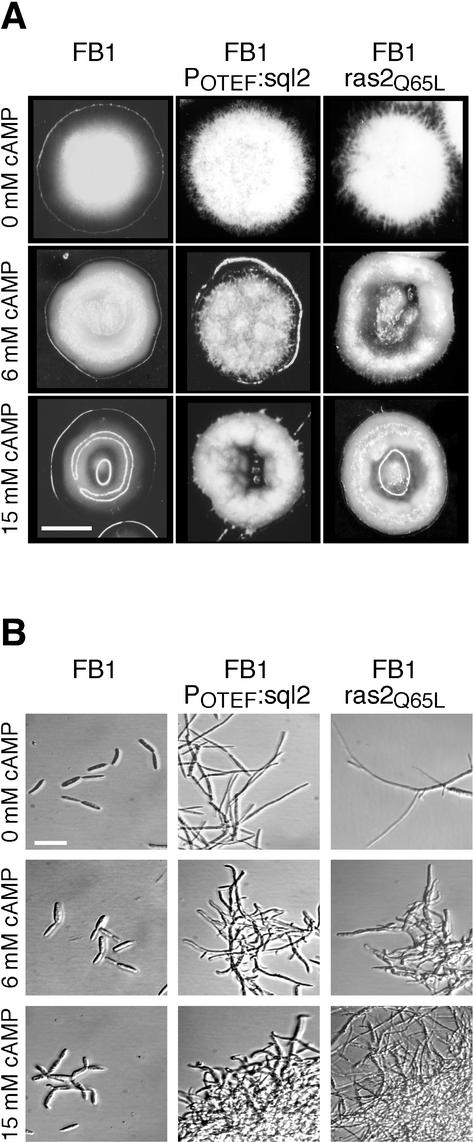
Since overexpression of sql2 in strains harboring gpa3Q206L resulted in an altered cell morphology, we have studied the effects of sql2 overexpression in a wild-type background. To this end, we replaced the endogenous sql2 gene in FB1 by POTEF:sql2, resulting in strain FB1POTEF:sql2. Colonies of FB1POTEF:sql2 displayed filamentous growth (Fig. 3A). In liquid media, aggregates of cells were observed, which were elongated and branched (Fig. 3B). This phenotype resembles the filamentous growth of strains with deletion of adenylyl cyclase (Uac1), which can be made to revert to wild-type morphology by addition of cAMP (28). Interestingly, in the presence of 6 mM cAMP, filament formation of FB1POTEF:sql2 on plates was significantly reduced (Fig. 3A), while in liquid medium containing 6 mM cAMP, FB1POTEF:sql2 still showed elongated cells (Fig. 3B). However, under these conditions, cell aggregates became denser and filaments were shorter, more branched, and slightly curved (Fig. 3B). These cell aggregates were reminiscent of those observed in FB1gpa3QLPOTEF:sql2 (Fig. 1B). In the presence of 15 mM cAMP, the wild-type strain FB1 started to grow by multiple budding as described previously (28), while in FB1POTEF:sql2, branching became more prominent and aggregation increased (Fig. 3B). These results show that overexpression of sql2 in wild-type strains induces filamentous growth, which cannot be made to revert to budding growth by addition of exogenous cAMP.
FIG. 3.
Overexpression of sql2 and dominant-active ras2Q65L induces filamentous growth. (A) Strains indicated at the top were grown on PD-charcoal plates with 0 mM cAMP (top panel), 6 mM cAMP (middle panel), or 15 mM cAMP (bottom panel). Bar, 1 mm. (B) Strains indicated at the top were grown in PD liquid medium with 0 mM cAMP (top panel), 6 mM cAMP (middle panel) or 15 mM cAMP (bottom panel). Bar, 20 μm.
To analyze whether filamentous growth induced by sql2 interferes with pathogenic development, we inoculated corn plants with crossings of FB1POTEF:sql2 and FB2POTEF:sql2. Interestingly, 83% of infected plants developed tumors, demonstrating that overexpression of sql2 does not affect pathogenicity (Table 1).
Isolation of two genes encoding Ras homologues.
Ras proteins are known to be the main effector proteins of CDC25-like GEFs. Therefore, it was plausible that overexpression or deletion of sql2 could influence the activity of Ras proteins. To further elucidate the role of Sql2 in U. maydis, we decided to isolate genes coding for Ras proteins.
Using two different short sequence tags showing homology to ras genes (kindly provided by Peter Margolis, Versicor, Inc., Fremont, Calif.), we cloned the ras1 and ras2 genes of U. maydis (see Materials and Methods). Sequencing of the obtained ras1 clone revealed that the ORF of ras1 was interrupted by a putative intron of 149 bp. Reverse transcription-PCR analysis confirmed the presence of this intron (data not shown). The ras1 mRNA codes for a putative protein of 215 amino acids. Sequence analysis of the ras2 clone indicated that the ras2 ORF was not disrupted by introns and codes for a putative protein of 192 amino acids. BlastX (2) analysis of ras1 and ras2 confirmed that both genes code for proteins with significant homology to other fungal Ras proteins. The deduced proteins have 48% overall identity, which is most prominent in their N-terminal domains. With respect to other fungal Ras proteins, U. maydis Ras1 shows high identity to Ras1 proteins from other organisms, e.g., 79% identity to Ras1 of S. pombe, 77% identity to Ras1 of Cryptococcus neoformans, and 75% identity to Ras1p of S. cerevisiae. Ras2 has moderate identity to other Ras2 proteins; e.g., 65% identity to Ras-2 of Neurospora crassa, 56% identity to Ras2p of S. cerevisiae, and 51% identity to Ras2 of C. neoformans. Ras1 and Ras2 from U. maydis have a conserved GTP-binding site and a C-terminal CAAX motif commonly found in small GTP-binding proteins.
The dominant-active allele of ras2 triggers filament formation.
To analyze the role of Ras2 in cell morphology, we constructed dominant-active alleles of ras2 by introducing a point mutation leading to the amino acid substitution Q65L (see Materials and Methods). It was shown that this kind of substitution causes the loss of intrinsic GTPase activity and results in a dominant-active Ras protein (66). To express ras2Q65L in U. maydis, we introduced this allele under the control of the native promoter into wild-type strain FB2 (see Materials and Methods). We selected three independent transformants that carried one copy of the construct at different sites in the genome (data not shown). All three FB2ras2Q65L mutants displayed filamentous growth on plates as well as in liquid media and were indistinguishable from each other (Fig. 3, shown for FB2ras2Q65L#5), indicating that this phenotype was due to the dominant-active allele of ras2. Filament formation of FB2ras2Q65L was strongly reduced when cAMP was added at a final concentration of 6 mM (Fig. 3A). In the presence of 15 mM cAMP, colonies of FB2ras2Q65L appeared glossy on the plates (Fig. 3A). In liquid medium, 6 mM cAMP induced branching and curving in filaments induced by ras2Q65L (Fig. 3B). In liquid medium containing 15 mM cAMP, FB2ras2Q65L cells formed clusters of branched filaments (Fig. 3B). Thus, the morphology of FB2ras2Q65L resembled the phenotype of FB1POTEF:sql2 (Fig. 3B). However, while both strains responded to exogenous cAMP, the amount of cAMP needed to induce the transitions was different: single colonies of FB2ras2Q65L appeared like doughnuts in the presence of 6 mM cAMP, while FB1POTEF:sql2 cells formed colonies of this type only with 15 mM cAMP added (Fig. 3A). In addition, exogenous cAMP (15 mM) induced the glossy colony phenotype in FB2ras2Q65L but did not do so in FB1POTEF:sql2 (Fig. 3A). These differences could be explained by assuming that expression of the dominant-active allele leads to higher levels of active Ras2 compared to active Ras2 levels attained by sql2 overexpression. On these grounds, we suggest that Sql2 might be the GEF that activates Ras2.
The dominant-active allele of ras1 induces mfa1 gene transcription.
To analyze the function of Ras1 in cell morphology, we constructed a dominant-active allele of ras1 by analogy to the ras2Q65L allele. This ras1Q67L allele was placed under the control of the crg1 promoter and introduced into the ip locus of FB1 in single copy (12). The crg1 promoter is repressed by glucose and induced by arabinose. In glucose-containing media, the resulting strain, FB1Pcrg1:ras1Q67L, showed wild-type morphology (data not shown). After a shift to arabinose-containing medium, no morphological changes in FB1Pcrg1:ras1Q67L were observed even after incubation for up to 16 h (data not shown). The absence of an influence on cell morphology suggests that Ras1 is not an effector of Sql2.
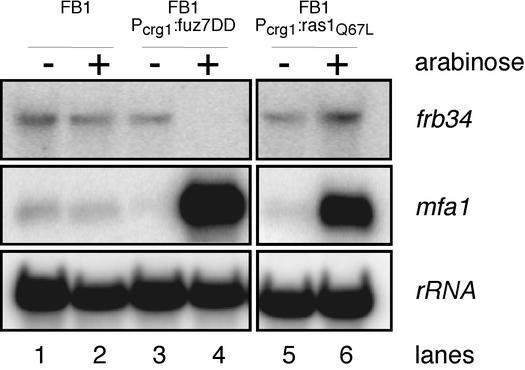
Since Ras proteins are known to regulate signaling pathways by interacting with proteins, which contains a so-called Ras association (RA) domain, we searched for proteins containing a putative RA domain. By this computer algorithm-based search, we identified at least three known proteins: the adenylyl cyclase Uac1 (amino acids 970 to 1050), the MAPKK kinase (MAPKKK) kinase Ubc4 (amino acids 44 to 153), and the adaptor protein Ubc2 (amino acids 402 to 490), which most probably acts upstream of Ubc4 (47). Since the cAMP cascade and the MAPK cascade both regulate pheromone gene (mfa1) expression, we analyzed whether expression of the ras1Q67L allele affects the level of mfa1 transcription. After inducing the expression of ras1Q67L, we detected elevated levels of mfa1 mRNAs (Fig. 4, lanes 5 and 6), whereas in the progenitor strain FB1, the levels of mfa1 transcription were unaffected by growth in arabinose-containing medium (lanes 1 and 2). Since mfa1 transcription is induced by activated cAMP as well as MAPK signaling (40; Müller et al., submitted), we analyzed whether the increased mfa1 expression triggered by ras1Q67L is due to activation of the cAMP cascade or the MAPK module. For this purpose, we chose a second reporter gene, frb34, encoding a putative acyltransferase, whose transcription is slightly elevated in the presence of high intracellular cAMP conditions (15). To analyze how this gene responds to an activated MAPK module, we used a strain that expresses a constitutively active allele of the MAPKK gene fuz7 (fuz7DD) under control of the crg1 promoter. After induction of fuz7DD, frb34 transcription was repressed (Fig. 4, lanes 3 and 4), whereas in the progenitor strain FB1, the expression of frb34 was not affected by the shift from glucose- to arabinose-containing medium (lanes 1 and 2). Thus, frb34 is a suitable reporter to distinguish between an active cAMP or MAPK cascade. When frb34 mRNA levels were compared after induction of ras1Q67L, a twofold increase was detected (lanes 5 and 6). Thus, ras1Q67L presumably activates the cAMP-signaling cascade to regulate mfa1 and frb34 gene expression.
FIG. 4.
Dominant-active ras1Q67L elevates pheromone gene expression. Strains indicated at the top were incubated in CM containing glucose (−) and then for 5 h in arabinose (+) as the carbon source. A 15-μg portion of total RNA was loaded per lane. The same filter was hybridized in succession with probes for mfa1, frb34, and rRNA as the loading control.
DISCUSSION
In this study, we identified sql2 as a multicopy suppressor of the glossy colony phenotype of a gpa3Q206L strain, which is supposed to have an activated cAMP pathway. sql2 encodes a structural homologue of CDC25-like GEFs. In particular, the C-terminal region of Sql2 contains one RasGEFN domain and RasGRF_CDC25 domain, which are known to catalyze the guanine nucleotide exchange in small G-proteins called Ras proteins (9, 13). In S. pombe, two different CDC25-like GEFs, Ste6 and Efc25, are present and have different functions. Deletion of ste6 abolishes conjugation, while deletion of efc25 affects cell morphology (34, 62). As in S. pombe, the CDC25 homologue of C. albicans is not essential for growth but is needed for the dimorphic switch in response to serum (24). In S. cerevisiae, two related CDC25-like GEFs exist: Cdc25p and Sdc25p, both of which have Ras GDP-GTP exchange activity (14, 20). While deletion of SDC25 results in no obvious phenotype, CDC25 is essential for growth by acting upstream of Ras1p and Ras2p (21, 38, 55, 60). In S. cerevisiae, cdc25 strains are viable when exogenous cAMP is provided, which demonstrates that death of cdc25 strains is due to dysfunction of the adenylyl cyclase Cyr1p (17).
In U. maydis, the Cdc25p homologue Sql2 differs in function from its homologues Cdc25p of S. cerevisiae and Ste6 of S. pombe in being dispensable for vegetative growth and conjugation. Δsql2 strains show no defect in plate mating assays, demonstrating that sql2 is not necessary for cell fusion and subsequent development of dikaryotic hyphae. However, Sql2 is required for full virulence, indicating that it is specifically involved in signaling processes activated during pathogenic development of U. maydis.
What are the signaling processes mediated by Sql2?
The findings that overexpression of sql2 suppresses the glossy colony appearance of gpa3Q206L strains and, conversely, that deletion of sql2 results in a glossy colony phenotype suggest that Sql2 influences cAMP signaling negatively. In addition, dikaryon development of Δsql2 mutants is more sensitive to cAMP than is that of the wild type. Consistent with a negative effect on cAMP signaling, sql2 overexpression triggers filamentous growth in haploid strains, which is also observed in strains affected in cAMP signaling. These results suggest that Sql2 could inhibit cAMP signaling directly. However, we consider it more likely that Sql2 acts in a pathway that operates in parallel to cAMP signaling. First, suppression of the glossy colony appearance of gpa3Q206L strains by sql2 is associated with a dramatic change in cell morphology, and overexpression of sql2 fails to suppress the characteristic tumor phenotype of gpa3Q206L strains. Thus, in the strict sense, sql2 cannot be classified as a genuine suppressor, which should have caused the mutant phenotype to revert to the wild type. Second, overexpression of sql2 does not interfere with pathogenic development, while strains affected in cAMP signaling are nonpathogenic (23). Third, the filamentous phenotype induced by sql2 is affected by adding cAMP but cannot be made to revert to the budding phenotype seen in wild-type strains. We take this to indicate that sql2 regulates morphology independently of the cAMP pathway, most probably via a pathway that is interconnected.
CD25-like GEFs are known to activate Ras proteins by catalyzing the GDP-GTP exchange in these proteins. In this study, we isolated two related genes, ras1 and ras2 (48% identity at the amino acid level), encoding proteins that are similar to known fungal Ras proteins. In U. maydis, the mRNA levels of ras1 and ras2 are comparable (data not shown). Two different Ras genes also exist in S. cerevisiae, both of which serve overlapping as well as distinct functions. In yeast, Ras2p and Ras1p function upstream of the adenylyl cyclase and only Ras2p also acts on the STE20/STE11/STE7/KSS1-MAPK module to regulate pseudohyphal differentiation (26, 48, 53). In S. pombe, Ras1 activates the pheromone-responsive MAPK cascade during conjugation and also regulates cell morphology independently of the MAPK cascade (25, 67). Recent studies revealed that these dual roles of Ras1 are controlled mainly by the action of two different Cdc25-like GEFs, Ste6 and Efc25. While Ste6 couples Ras1 to the MAPK pathway, Efc25 links Ras1 to the Cdc42 pathway (52). In U. maydis, the two different Ras proteins seem to operate in different signaling pathways. Expression of a dominant-active allele of ras2 induces filaments, while expression of a dominant-active allele of ras1 does not affect cell morphology but induces pheromone gene expression. In contrast to an activated MAPK module which represses the expression of frb34, this reporter gene is induced twofold by ras1Q67L. We take this as an indication that Ras1 activates the cAMP pathway. It has been shown that in S. cerevisiae, dominant-active Ras2p activates Cyr1p and binds to a RA domain found in the leucine-rich repeats of Cyr1p (19, 39, 61). The adenylyl cyclase, Uac1, of U. maydis also harbors a RA domain (amino acids 970 to 1050), and Ras1 may be coupled to Uac1 by interaction via this domain.
The second Ras protein in U. maydis, Ras2, is unlikely to act on adenylyl cyclase, since the filaments developed by strains expressing ras2Q65L are clearly distinct from the filaments induced under low-cAMP conditions. In addition, the filaments induced by ras2Q65L are similar to the filaments triggered by overexpression of sql2. Therefore, we assume that Sql2 may be the activator of Ras2. Consistently, yeast two-hybrid studies show that Sql2 interacts weakly with Ras2 but fails to interact with Ras1 (J. Katzenberger and R. Kahmann, unpublished data). Therefore, it is conceivable that Ras1 is activated by another, not yet identified, GEF.
The signaling cascade by which Sql2 and Ras2 regulate cell morphology in U. maydis is not known. One candidate effector pathway may include a Cdc42 homologue. In S. pombe, Cdc42 is regulated by Ras1 and acts on the Pak1/Shk1 kinase involved in polarized growth, presumably by affecting the cytoskeleton. In particular, it was shown that overexpression of dominant-negative Pak1 results is delocalized actin (51). These and other examples suggest a direct link between Ras signaling and the cytoskeleton in fungi (1, 16, 31, 65) that is critical for morphogenesis. Hence, for the situation in U. maydis, it is conceivable that Sql2 and Ras2 take part in controlling morphogenesis by regulating the cytoskeleton.
Alternatively, Ras2 in U. maydis may act on the MAPK cascade composed of Ubc4, Fuz7, and Kpp2/Ubc3, since this cascade is a positive regulator of filamentous growth. Moreover, the farthest-upstream component, Kpp4/Ubc4, contains a RA domain that is conserved in proteins interacting with Ras proteins (3; Müller et al., submitted). In S. pombe, Ras1 regulates the Ubc4 homologue, Byr2, by interacting with the RA domain of Byr2 MAPKK kinase, and this interaction leads to the translocation of Byr2 to the plasma membrane (8, 45, 63). Recently, the ras2 gene of U. maydis was found to act upstream of kpp2/ubc3 (42). Mutants lacking ras2 are attenuated in mating and impaired in pathogenic development (42). To reconcile this with our data that deletion of sql2 affects pathogenicity only, one would have to propose that Sql2 might control Ras2 activity specifically during pathogenic development. For future studies, it will be very interesting to elucidate which signals are transmitted to Sql2 and to determine how and at which stage Ras2 becomes activated by Sql2 or other effectors.
Acknowledgments
P. Müller and J. Katzenberger contributed equally to the work.
We are most grateful to Peter Margolis (Versicor, Inc., Fremont, Calif.) for providing the two sequence tags of ras genes. We thank Jan Schirawski for his critical comments on the manuscript.
This work was supported by the DFG through SFB369. G.L. was supported by a Marie Curie postdoctoral training grant from the European Commission.
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, D. L., J. D. Egan, M. E. Mayorga, and S. E. Gold. 2000. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol. Plant-Microbe Interact. 13:781-786. [DOI] [PubMed] [Google Scholar]
- 4.Banuett, F., and I. Herskowitz. 1989. Different a alleles are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 86:5878-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banuett, F., and I. Herskowitz. 1996. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122:2965-2976. [DOI] [PubMed] [Google Scholar]
- 6.Banuett, F., and I. Herskowitz. 1994. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 8:1367-1378. [DOI] [PubMed] [Google Scholar]
- 7.Barrett, K. J., S. E. Gold, and J. W. Kronstad. 1993. Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol. Plant-Microbe Interact. 6:274-283. [DOI] [PubMed] [Google Scholar]
- 8.Bauman, P., Q. C. Cheng, and C. F. Albright. 1998. The Byr2 kinase translocates to the plasma membrane in a Ras1-dependent manner. Biochem. Biophys. Res. Commun. 244:468-474. [DOI] [PubMed] [Google Scholar]
- 9.Boguski, M. S., and F. McCormick. 1993. Proteins regulating Ras and its relatives. Nature 366:643-654. [DOI] [PubMed] [Google Scholar]
- 10.Bolker, M., H. U. Bohnert, K. H. Braun, J. Gorl, and R. Kahmann. 1995. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol. Gen. Genet. 248:547-552. [DOI] [PubMed] [Google Scholar]
- 11.Bolker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 12.Bottin, A., J. Kamper, and R. Kahmann. 1996. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 253:342-352. [DOI] [PubMed] [Google Scholar]
- 13.Bourne, H. R., D. A. Sanders, and F. McCormick. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349:117-127. [DOI] [PubMed] [Google Scholar]
- 14.Boy-Marcotte, E., A. Buu, C. Soustelle, P. Poullet, A. Parmeggiani, and M. Jacquet. 1993. The C-terminal part of the CDC25 gene product has Ras-nucleotide exchange activity when present in a chimeric SDC25-CDC25 protein. Curr. Genet. 23:397-401. [DOI] [PubMed] [Google Scholar]
- 15.Brachmann, A., G. Weinzierl, J. Kamper, and R. Kahmann. 2001. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42:1047-1063. [DOI] [PubMed] [Google Scholar]
- 16.Cali, B. M., T. C. Doyle, D. Botstein, and G. R. Fink. 1998. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 9:1873-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camonis, J. H., M. Kalekine, B. Gondre, H. Garreau, E. Boy-Marcotte, and M. Jacquet. 1986. Characterization, cloning and sequence analysis of the CDC25 gene which controls the cyclic AMP level of Saccharomyces cerevisiae. EMBO J. 5:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen, J. J. 1963. Corn smut induced by Ustilago maydis. Am. Phytopathol. Soc. Monogr. 2:5-41. [Google Scholar]
- 19.Colicelli, J., J. Field, R. Ballester, N. Chester, D. Young, and M. Wigler. 1990. Mutational mapping of RAS-responsive domains of the Saccharomyces cerevisiae adenylyl cyclase. Mol. Cell. Biol. 10:2539-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crechet, J. B., P. Poullet, M. Y. Mistou, A. Parmeggiani, J. Camonis, E. Boy-Marcotte, F. Damak, and M. Jacquet. 1990. Enhancement of the GDP-GTP exchange of RAS proteins by the carboxyl-terminal domain of SCD25. Science 248:866-868. [DOI] [PubMed] [Google Scholar]
- 21.Damak, F., E. Boy-Marcotte, D. Le-Roscouet, R. Guilbaud, and M. Jacquet. 1991. SDC25, a CDC25-like gene which contains a RAS-activating domain and is a dispensable gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 11:202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Souza, C. A., and J. Heitman. 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25:349-364. [DOI] [PubMed] [Google Scholar]
- 23.Durrenberger, F., K. Wong, and J. W. Kronstad. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 95:5684-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enloe, B., A. Diamond, and A. P. Mitchell. 2000. A single-transformation gene function test in diploid Candida albicans. J. Bacteriol. 182:5730-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukui, Y., T. Kozasa, Y. Kaziro, T. Takeda, and M. Yamamoto. 1986. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell 44:329-336. [DOI] [PubMed] [Google Scholar]
- 26.Gancedo, J. M. 2001. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:107-123. [DOI] [PubMed] [Google Scholar]
- 27.Gillissen, B., J. Bergemann, C. Sandmann, B. Schroeer, M. Bolker, and R. Kahmann. 1992. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68:647-657. [DOI] [PubMed] [Google Scholar]
- 28.Gold, S., G. Duncan, K. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 29.Gold, S. E., S. M. Brogdon, M. E. Mayorga, and J. W. Kronstad. 1997. The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 9:1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann, H. A., R. Kahmann, and M. Bolker. 1996. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 15:1632-1641. [PMC free article] [PubMed] [Google Scholar]
- 31.Ho, J., and A. Bretscher. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12:1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 33.Holliday, R. 1974. Ustilago maydis, p. 575-595. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Press, New York, N.Y.
- 34.Hughes, D. A., Y. Fukui, and M. Yamamoto. 1990. Homologous activators of ras in fission and budding yeast. Nature 344:355-357. [DOI] [PubMed] [Google Scholar]
- 35.Isshiki, T., N. Mochizuki, T. Maeda, and M. Yamamoto. 1992. Characterization of a fission yeast gene, gpa2, that encodes a G alpha subunit involved in the monitoring of nutrition. Genes Dev. 6:2455-2462. [DOI] [PubMed] [Google Scholar]
- 36.Kamper, J., M. Reichmann, T. Romeis, M. Bolker, and R. Kahmann. 1995. Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81:73-83. [DOI] [PubMed] [Google Scholar]
- 37.Kataoka, T., D. Broek, and M. Wigler. 1985. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell 43:493-505. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka, T., S. Powers, C. McGill, O. Fasano, J. Strathern, J. Broach, and M. Wigler. 1984. Genetic analysis of yeast RAS1 and RAS2 genes. Cell 37:437-445. [DOI] [PubMed] [Google Scholar]
- 39.Kido, M., F. Shima, T. Satoh, T. Asato, K. Kariya, and T. Kataoka. 2002. Critical function of the Ras-associating domain as a primary Ras- binding site for regulation of Saccharomyces cerevisiae adenylyl cyclase. J. Biol. Chem. 277:3117-3123. [DOI] [PubMed] [Google Scholar]
- 40.Kruger, J., G. Loubradou, E. Regenfelder, A. Hartmann, and R. Kahmann. 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260:193-198. [DOI] [PubMed] [Google Scholar]
- 41.Kruger, J., G. Loubradou, G. Wanner, E. Regenfelder, M. Feldbrugge, and R. Kahmann. 2000. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol. Plant-Microbe Interact. 13:1034-1040. [DOI] [PubMed] [Google Scholar]
- 42.Lee, N., and J. W. Kronstad. 2002. ras2 controls morphogenesis, pheromone response, and pathogenicity in the fungal pathogen Ustilago maydis. Eukaryot. Cell 1:954-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letunic, I., L. Goodstadt, N. J. Dickens, T. Doerks, J. Schultz, R. Mott, F. Ciccarelli, R. R. Copley, C. P. Ponting, and P. Bork. 2002. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic. Acids Res. 30:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loubradou, G., A. Brachmann, M. Feldbrugge, and R. Kahmann. 2001. A homologue of the transcriptional repressor Ssn6p antagonizes cAMP signalling in Ustilago maydis. Mol. Microbiol. 40:719-730. [DOI] [PubMed] [Google Scholar]
- 45.Masuda, T., K. Kariya, M. Shinkai, T. Okada, and T. Kataoka. 1995. Protein kinase Byr2 is a target of Ras1 in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 270:1979-1982. [DOI] [PubMed] [Google Scholar]
- 46.Mayorga, M. E., and S. E. Gold. 1999. A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34:485-497. [DOI] [PubMed] [Google Scholar]
- 47.Mayorga, M. E., and S. E. Gold. 2001. The ubc2 gene of Ustilago maydis encodes a putative novel adaptor protein required for filamentous growth, pheromone response and virulence. Mol. Microbiol. 41:1365-1379. [DOI] [PubMed] [Google Scholar]
- 48.Mosch, H. U., R. L. Roberts, and G. R. Fink. 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller, P., C. Aichinger, M. Feldbrugge, and R. Kahmann. 1999. The MAP kinase kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol. Microbiol. 34:1007-1017. [DOI] [PubMed] [Google Scholar]
- 50.Nakafuku, M., T. Obara, K. Kaibuchi, I. Miyajima, A. Miyajima, H. Itoh, S. Nakamura, K. Arai, K. Matsumoto, and Y. Kaziro. 1988. Isolation of a second yeast Saccharomyces cerevisiae gene (GPA2) coding for guanine nucleotide-binding regulatory protein: studies on its structure and possible functions. Proc. Natl. Acad. Sci. USA 85:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottilie, S., P. J. Miller, D. I. Johnson, C. L. Creasy, M. A. Sells, S. Bagrodia, S. L. Forsburg, and J. Chernoff. 1995. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 14:5908-5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papadaki, P., V. Pizon, B. Onken, and E. C. Chang. 2002. Two ras pathways in fission yeast are differentially regulated by two ras guanine nucleotide exchange factors. Mol. Cell. Biol. 22:4598-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peter, M., A. M. Neiman, H. O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 54.Regenfelder, E., T. Spellig, A. Hartmann, S. Lauenstein, M. Bolker, and R. Kahmann. 1997. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 16:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson, L. C., J. B. Gibbs, M. S. Marshall, I. S. Sigal, and K. Tatchell. 1987. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science 235:1218-1221. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Frisch, and T. Maniatis 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz, B., F. Banuett, M. Dahl, R. Schlesinger, W. Schäfer, T. Martin, I. Herskowitz, and R. Kahmann. 1990. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60:295-306. [DOI] [PubMed] [Google Scholar]
- 59.Spellig, T., A. Bottin, and R. Kahmann. 1996. Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol. Gen. Genet. 252:503-509. [DOI] [PubMed] [Google Scholar]
- 60.Tatchell, K., D. T. Chaleff, D. DeFeo-Jones, and E. M. Scolnick. 1984. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature 309:523-527. [DOI] [PubMed] [Google Scholar]
- 61.Toda, T., I. Uno, T. Ishikawa, S. Powers, T. Kataoka, D. Broek, S. Cameron, J. Broach, K. Matsumoto, and M. Wigler. 1985. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27-36. [DOI] [PubMed] [Google Scholar]
- 62.Tratner, I., A. Fourticq-Esqueoute, J. Tillit, and G. Baldacci. 1997. Cloning and characterization of the S. pombe gene efc25+, a new putative guanine nucleotide exchange factor. Gene 193:203-210. [DOI] [PubMed] [Google Scholar]
- 63.Tu, H., M. Barr, D. L. Dong, and M. Wigler. 1997. Multiple regulatory domains on the Byr2 protein kinase. Mol. Cell. Biol. 17:5876-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban, M., R. Kahmann, and M. Bolker. 1996. Identification of the pheromone response element in Ustilago maydis. Mol. Gen. Genet. 251:31-37. [DOI] [PubMed] [Google Scholar]
- 65.Waugh, M. S., C. B. Nichols, C. M. DeCesare, G. M. Cox, J. Heitman, and J. A. Alspaugh. 2002. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology 148:191-201. [DOI] [PubMed] [Google Scholar]
- 66.Wiseman, R. W., S. J. Stowers, E. C. Miller, M. W. Anderson, and J. A. Miller. 1986. Activating mutations of the c-Ha-ras protooncogene in chemically induced hepatomas of the male B6C3 F1 mouse. Proc. Natl. Acad. Sci. USA 83:5825-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu, H. P., M. White, S. Marcus, and M. Wigler. 1994. Concerted action of RAS and G proteins in the sexual response pathways of Schizosaccharomyces pombe. Mol. Cell. Biol. 14:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]