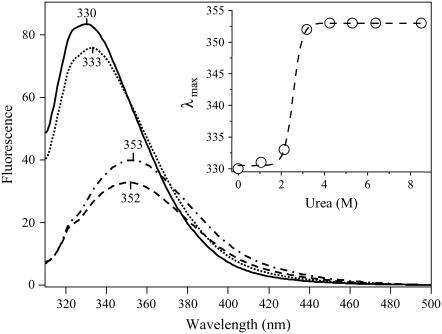
FIGURE 5.
Urea titration of DTA (C186A mutant) monitored by tryptophan fluorescence. The exposure of DTA to increasing amounts of urea from 0 M to 8.5 M results in both a decrease in the fluorescence intensity and a red-shift of the emission maximum of the tryptophan fluorescence spectrum; both effects are consistent with the exposure of tryptophan residues to aqueous solvent because of the unfolding of DTA. Shown are illustrative spectra of DTA in 0 M (solid), 2.13 M (dots), 3.18 M (dash), and 8.50 M (dot-dash) urea. (Inset) Plot of the emission maximum as a function of urea concentration (open circles). The data are described (R2 = 0.999) by a sigmoidal function (dashed line) indicative of a two-state unfolding process. The midpoint of the transition is 2.6 M.