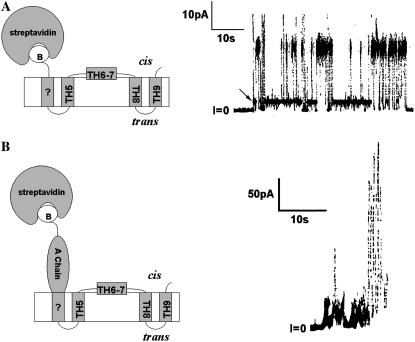
FIGURE 7.
Channel behavior when the N-terminus of either the T domain (A) or A-T (B) is forced to remain on the cis side. A cartoon for each of the resulting states is shown on the left side of the panel. (A) Channel formed by the T domain with streptavidin bound to the biotinylated cysteine in the N-terminal His6-tag. The cis solution contained 0.3 μg/ml of T domain whose N-terminal His6-tag was biotinylated and had been preincubated with streptavidin. Note the large size of this channel. (The arrow marks a substate having the same conductance under the pH conditions of the experiment as that of a channel without streptavidin attached. We speculate that the switches to a smaller conductance level seen in this trace result from the lateral movement within the bilayer of the fourth transmembrane segment away from the others, thereby generating a “normal” three-transmembrane-segment channel.) The topography of TH5 to TH9 in the cartoon is that of the “normal” channel (15); in the “normal” channel, everything N-terminal to TH5 resides on the trans side (Fig. 1). The “B” in the cartoon stands for biotin. The solutions on both the cis and trans sides of the membrane contained 1 M KC1, 2 mM CaC12, and 1 mM EDTA; the cis solution also contained 30 mM MES, pH 5.3, and the trans solution contained 50 mM HEPES, pH 7.2. The applied voltage is +60 mV. (From Gordon (33).) (B) Activity generated by A-T with streptavidin bound to a biotin at the N-terminus of the A chain. The cis solution contained 35 ng/ml A-T biotinylated at its N-terminus and 20 μg/ml streptavidin that was present before the addition of A-T. Note the erratic current fluctuations and lack of a clear channel conductance state. The membrane separated symmetric solutions of 1 M KC1, 5 mM CaC12, 1 mM EDTA, 20 mM potassium malate, pH 4.85. The applied voltage is +60 mV.