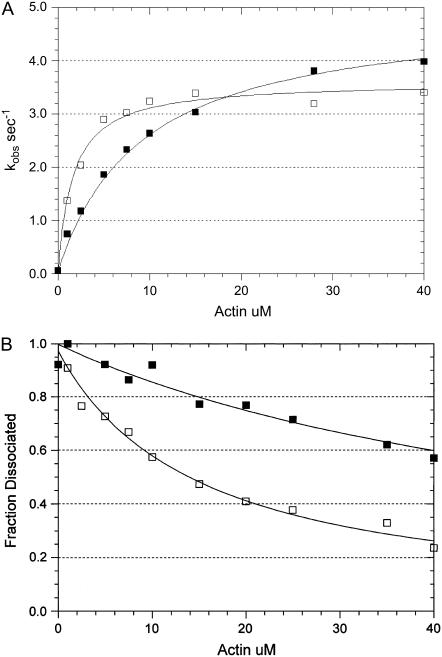
FIGURE 11.
Effect of PEG on actin-activated ATP hydrolysis and the steady-state binding of myosin to actin in the presence of ATP. (A) The actin dependence of the rate of steady-state ATP hydrolysis. Experimental conditions: 0.1 μM rabbit skeletal myosin-S1A1, 5 mM MOPS, 2 mM MgCl2, 20 mM KAc, pH 7, 20°C, 1 mM ATP, the indicated actin concentration, and either zero (solid squares) or 5 percent (w/v) 1000 Da PEG (open squares). The data sets were fit to a hyperbolic equation: V = Vmax/(1 + Kapp/[actin]) where Vmax = 4.9 s−1 and Kapp = 9.2 μM in the absence of PEG and Vmax = 3.6 s−1 and Kapp = 1.6 μM in the presence of PEG. (B) The actin dependence of the fraction of myosin-S1A1 remaining in the supernatant after centrifugation in the presence of the indicated concentration of actin. The data sets were fit to a hyperbolic equation: f = 1 − (1/(1 + Kactin/[actin])) where f is the fraction dissociated and Kactin = 60 μM in the absence of PEG (solid squares) and Kactin = 15 μM in the presence of PEG (open squares). The experimental conditions are the same as those in A except that the concentration of myosin-S1A1 was 0.01 μM.