Abstract
The use of the phospholipid fatty acid (PLFA) composition of microorganisms to detect previous self-heating events was studied in naturally self-heated peat and in peat incubated under temperature-controlled conditions. An increased content of total PLFAs was found in self-heated peat compared to that in unheated peat. Two PLFAs, denoted T1 and T2, were detected only in the self-heated peat. Incubation of peat samples at 25 to 55°C for 4 days indicated that T1 and T2 were produced from microorganisms with different optimum temperatures. This was confirmed by isolation of bacteria at 55°C, which produced T2 but not T1. These bacteria produced another PLFA (denoted T3) which coeluted with 18:1ω7. T2 and T3 were identified as ω-cyclohexyltridecanoic acid and ω-cyclohexylundecanoic acid, respectively, indicating that the bacteria belonged to the genus Alicyclobacillus. T1 was tentatively identified as ω-cycloheptylundecanoic acid. T2 was detected 8 h after the peat incubation temperature was increased to 55°C, and maximum levels were found within 5 days of incubation. The PLFA 18:1ω7-T3 increased in proportion to T2. T1 was detected after 96 h at 55°C, and its level increased throughout the incubation period, so that it eventually became one of the dominant PLFAs after 80 days. In peat samples incubated at 55°C and then at 25°C, T1 and T2 disappeared slowly. After 3 months, detectable levels were still found. Incubation at 25°C after heating for 3 days at 55°C decreased the amounts of T2 and 18:1ω7-T3 faster than did incubation at 5°C. Thus, not only the duration and temperature during the heating event but also the storage temperature following heating are important for the detection of PLFAs indicating previous self-heating.
Peat is widely used as organic growth substrate in horticulture. Properties like a high cation exchange capacity, a high proportion of air-filled pores, and a low content of weeds and pathogens makes it an ideal growth medium for plant production. After harvesting, the peat is stored in stockpiles. During this storage, self-heating may occur. The use of self-heated peat is generally avoided, since toxic substances produced during self-heating may inhibit seed germination and plant growth (24). Test methods have been developed to detect if peat has been exposed to self-heating. These tests are based on biological, chemical (13), or physical (20) analyses. However, they require access to unheated peat samples for comparison, which make the tests cumbersome, and today they are no longer in use.
The self-heating process in peat has not been well studied, but it is assumed to be analogous to self-heating in compost and hay (9). During self-heating, a succession from a mesophilic to a thermophilic microbial community is expected (7, 18, 22). After the peak temperature is attained, there will be a selection pressure favoring a mesophilic community, and a reversion from a thermophilic to a mesophilic community will take place (21). Analyses of phospholipid fatty acids (PLFAs) have been used to detect these changes, e.g., in different types of composts (6, 11, 12, 16, 17). The initial thermophilic phase appeared to cause immediate and drastic changes in the PLFA pattern within a day, whereas changes during the later mesophilic curing phase were much slower. Even 3 to 12 months after the thermophilic phase, PLFAs characteristic of this phase were detected (6, 16). This indicates that the PLFA pattern might be used to detect self-heating of peat long after the actual event.
The objectives of the present study were (i) to determine if the PLFA analysis can be used as a method for detection of self-heating in peat and (ii) to study how mesophilic and thermophilic temperatures affected the microbial community structure of peat. This was done by studying naturally self-heated peat stored for up to 6 months after the heating event and peat incubated under temperature-controlled conditions.
MATERIALS AND METHODS
Peat samples.
Peat samples were collected from stockpiles containing ground sod peat or vacuum-harvested milled peat. The stockpiles were located on the mires where harvesting had taken place. All mires were situated in southern Norway and were dominated by different Sphagnum species with a low degree of humification (von Post H2 to H4). Sampling was done 4 to 6 months after harvesting. One sample per stockpile was analyzed. pH was measured in a peat-water suspension (1:1.5, vol/vol). NO3-N and NH4-N were measured after extraction in a 2 M KCl solution. Dissolved organic carbon (DOC) was measured in the water extract from the pH measurement. The C/N ratio was determined using dry combustion in an excess of oxygen, with thermal conductivity detection of evolved CO2 and N2O.
Peat treatments.
Mire and stockpile number are used to identify the peat samples. A single sample from a stockpile was used as a replicate for temperature-controlled laboratory experiments. Typically, two or three replicates were used per experiment; these are indicated in the descriptions of the experimental design. Before the peat samples were incubated, ultrapure Milli Q water was added to increase the water content of the peat to 80%. During the experiments, the samples were kept in autoclave bags and maintained at constant moisture content. The desired temperatures in the peat were reached within an hour.
To differentiate between abiotic and biotic effects of temperature, three peat samples (Bliksrud 3, Killingmo 2, and Komnes 3) were subjected to the following treatments: (i) autoclaving (121°C for 1 h), (ii) autoclaving followed by 3 days of incubation at 55°C, (iii) gamma irradiation (31 kGy, at the Norwegian Institute for Energy Technology), (iv) gamma irradiation followed by 3 days of incubation at 55°C, (v) incubation at 90°C for 3 days before PLFA measurements. Untreated peat and peat samples incubated for 3 days at 55°C were included for comparison.
The effect of different temperature regimens was studied using three peat samples (Bliksrud 3, Killingmo 2, and Komnes 2) incubated at 25, 35, 45, and 55°C. The PLFA pattern was determined after 4 days.
In a short-term study, the PLFA pattern was determined in two peat samples (Killingmo 2 and Komnes 2) incubated at 55°C for 96 h. Since sampling was done at short time intervals and to avoid temperature fluctuations, the peat samples were kept in numerous autoclave bags that corresponded to the number of samplings.
The effect of long-term high temperature was studied by using three peat samples (Gjødalsmosan 1, Killingmo 2, and Komnes 2) incubated at 55°C, with the last PLFA extraction performed after 80 days of incubation. After 3 and 11 days at 55°C, parts of these samples were moved to 25°C to study the reestablishment of the mesophilic community. In addition, two peat samples (Grenimosan 3 and Killingmo 2) were incubated for 3 days at 55°C and then transferred to 25, 15, and 5°C. These temperatures were selected to simulate the effect of different storage temperatures after a heating event. The effect on the PLFA pattern was monitored for 50 days.
Lipid extraction and PLFA analysis.
PLFAs were extracted and analyzed by a procedure described by Frostegård et al. (8), except that larger volumes of the solvents had to be used due to high absorption in the peat. Briefly, 2 g of peat was extracted in 21 ml of a one-phase mixture of chloroform-methanol-citrate buffer (1:2:0.8, vol/vol/vol). A 4-ml volume of chloroform and 4 ml of citrate buffer respectively were added to split the phases. The remaining procedure was as described previously (8).
Fatty acid nomenclature.
The fatty acid nomenclature used is as follows: total number of carbon atoms:number of double bonds, followed by the position (ω) of the double bond from the methyl end of the molecule. cis and trans configurations are indicated by c and t, respectively. Anteiso- and isobranching are designated by the prefix a or i. 10Me is a methyl group on the 10th carbon atom from the carboxyl end of the molecule. Cy indicates cyclopropane fatty acids. T1 and T2 denote PLFAs found after heating of peat. T3 denotes a PLFA coeluting with 18:1ω7 in heated peat samples. To indicate that peat contains a mixture of 18:1ω7 and T3, the notation 18:1ω7-T3 is used.
Pure-culture studies.
Thermophilic bacteria were isolated from heated peat samples on peat agar incubated at 55°C. Peat agar was prepared by autoclaving 100 g of peat in 1 liter of distilled water, followed by filtration. The peat extract (pH 3.8) was diluted in water (1:1) before preparation of the agar. Care was taken that the plates and the dilution liquid (diluted peat extract) were at 55°C when the plating was performed. All visible bacterial colonies appeared similar. Six isolates from different peat samples were picked at random, plated on peat agar-0.1% tryptic soy agar, and incubated at 25, 45, and 55°C for 1 week. The PLFA content of the six pure cultures was then determined by flooding the plates with citrate buffer. A 1.5-ml portion of the bacterial suspension was used for PLFA analyses.
Data analysis.
The moles percent of the individual PLFAs in each peat sample were subjected to principal-component analysis (PCA). To give each variable an equal chance to contribute to the variation, all data were weighted to unit variance before the PCA was performed, using the Unscrambler software (Camo A/S, Trondheim, Norway). When self-heated and unheated peat samples were compared (see Fig. 1), PLFAs of eucaryotic origin (presumably from plants and mosses [23]) were excluded from the analysis.
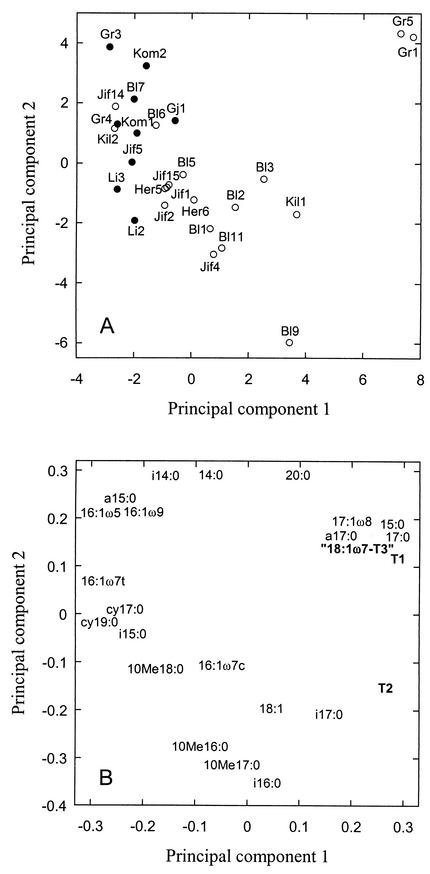
FIG. 1.
PCA of the PLFA pattern of self-heated (open symbols) and unheated (solid symbols) peat. Bl, Bliksrud; Gr, Grenimosan; Her, Herremyr; Kil, Killingmo; Jif, Jif peat; Gj, Gjødalsmosan; Kom, Komnes; Li, Liermosan. For background data, see Table 1. Samples denoted Jif2, Jif4, Jif14, and Jif15 were subjected only to PLFA analyses and are therefore not included in the table. (A) Score plot of the different peat samples. (B) Loading plot of the individual PLFAs. The PLFAs T1, T2, and 18:1ω7-T3 are in bold.
Differences between PLFAs in self-heated and unheated peat were tested by Student's t test. In the laboratory experiments, the basic replication unit to calculate standard errors was one sample from one stockpile. When analysis of variance (ANOVA) was used, the different peat samples were treated as blocks.
RESULTS
PLFAs in self-heated and unheated peat.
All peat samples were acidic, with C/N ratios ranging from 42 to 61 (Table 1). Naturally self-heated peat was defined as peat that had been exposed to temperatures above 35°C. The total amount of PLFAs (totPLFA) was significantly larger (P < 0.05) in the self-heated peat than in unheated peat (1,160 ± 99 [n = 18] and 760 ± 106 [n = 9] nmol g−1, respectively). Thus, the absolute amounts of many of the individual PLFAs were significantly larger (P < 0.05) in the self-heated peat than in the unheated peat (15 of 28 samples [data not shown]). In the self-heated peat, two fatty acids (denoted T1 and T2) not detected in any of the unheated peat samples were found. These two PLFAs usually appeared together, although sometimes only T2 was detected. T1 varied between 0 and 0.42 mol% of totPLFA (mean, 0.08%), and T2 varied between 0.08 and 0.66 mol% of totPLFA (mean, 0.30%) in the self-heated peat.
TABLE 1.
Chemical description of peat samples. NO3-N, NH4-N, and DOC
| Mire (stockpile no.) | pH | Amt (μg/g [dry wt] of peat) of:
|
C/N ratio | Temp (°C)a | ||
|---|---|---|---|---|---|---|
| NO3-N | NH4-N | DOC | ||||
| Bliksrud (1) | 3.9 | 6.0 | 128 | 2,590 | 46 | — (H)b |
| Bliksrud (2) | 3.9 | 7.0 | 79 | 5,490 | 43 | 42 (H) |
| Bliksrud (3) | 3.9 | 6.3 | 181 | 7,210 | 42 | 51 (H) |
| Bliksrud (5) | 3.6 | 7.6 | 81 | 4,970 | 48 | 46 (H) |
| Bliksrud (6) | 3.9 | 6.3 | 37 | 4,320 | 45 | 43 (H) |
| Bliksrud (7) | 4.0 | 9.5 | 50 | 2,490 | 51 | 13 (U) |
| Bliksrud (9) | 3.7 | 7.5 | 139 | 10,700 | 50 | 45 (H) |
| Bliksrud (11) | 3.8 | 7.3 | 159 | 4,430 | 47 | 35 (H) |
| Gjødalsmosan (1) | 4.2 (3.8)c | 17 (19) | 180 (445) | 4,170 (13,200) | 45 | — (U)d |
| Grenimosan (1) | 3.3 | 6.4 | 10 | 16,600 | NAe | 55 (H) |
| Grenimosan (3) | 4.0 | 18 | 203 | 1,950 | 51 | 15 (U) |
| Grenimosan (4) | 3.7 | 19 | 263 | 1,840 | 51 | 14 (U) |
| Grenimosan (5) | 3.4 | 2.4 | 15 | 10,300 | NA | 55 (H) |
| Herremyr (5) | 3.3 | 6.7 | 63 | 14,200 | 47 | 35 (H) |
| Herremyr (6) | 3.8 | 4.1 | 125 | 16,400 | 50 | 55 (H) |
| Jif peat (1) | 3.8 | 20 | 61 | 2,500 | 44 | 35 (H) |
| Jif peat (5) | 3.8 | 10 | 72 | 2,470 | 50 | 19 (U) |
| Killingmo (1) | 3.2 | 4.5 | 685 | 35,800 | 52 | 40 (H) |
| Killingmo (2) | 4.4 (4.0) | 25 (21) | 100 (200) | 3,160 (4,710) | 53 | 40 (H) |
| Komnes (1) | 3.7 | 7.5 | 76 | 10,200 | 56 | 33 (U) |
| Komnes (2) | 4.9 (4.9) | 4 (26) | 243 (312) | 2,020 (6,450) | 61 | 22 (U) |
| Liermosan (2) | 3.6 | 43 | 188 | 1,240 | 48 | 15 (U) |
| Liermosan (3) | 3.7 | 20 | 34 | 1,520 | NA | 30 (U) |
The temperature at sampling is specified. H, self-heated peat; U, unheated peat.
Stockpile was laid open.
Values in parentheses are those obtained after heating in the laboratory.
Sod peat that was ground directly before sampling.
NA, not analyzed.
A PCA score plot differentiated between self-heated and unheated peat samples (Fig. 1A). The self-heated peat samples were found mostly to the right of the first principal component, and the unheated peat samples were found mostly to the left. Of the total variation, 34% was accounted for by the first principal component while 24% was accounted for by the second principal component. There was a significant difference between the scores of self-heated and unheated peat samples along the first component (P < 0.01). The loading values of the individual PLFAs showed that unheated peat samples were characterized by relatively high proportions of the PLFAs cy19:0, cy17:0, 16:1ω5, 16:1ω7t, 16:1ω9c, a15:0, and i15:0, while the self-heated peat samples had relatively high proportions of the PLFAs T1, T2, 18:1ω7-T3, 15:0, 17:0, i17:0, and 17:1ω8 (Fig. 1B).
Effects on PLFA composition after autoclaving, gamma irradiation, and heating at 55 and 90°C.
Autoclaving, gamma irradiation, and incubation at 90°C (for 3 days) reduced the amounts of totPLFA compared to those in unheated control samples (Table 2). Incubation at 90°C reduced totPLFA the most. Subsequent incubation at 55°C for 3 days did not alter the amounts of totPLFA. Only the 55°C incubation of unsterilized peat resulted in the production of T1, T2, and 18:1ω7-T3, indicating that microorganisms produced these PLFAs. These PLFAs were not found in the controls, except for T1 in the Bliksrud sample, which had previously been subjected to self-heating.
TABLE 2.
Changes in the concentrations of PLFAs due to different sterilization and heat treatments compared to a control incubated at 25°C
| PLFA | Concn of PLFA (nmol/g [dry wt] of peat) fora:
|
||||||
|---|---|---|---|---|---|---|---|
| Control (25°C) | 55°C | Autoclaved | Autoclaved + 55°C | Gamma | Gamma + 55°C | 90°C | |
| T1 | 1.3 | 6.3 ± 1.7 | 0 | 0 | 0 | 0 | 0 |
| T2 | 0 | 13.7 ± 3.2 | 0 | 0 | 0 | 0 | 0 |
| 18:1ω7-T3 | 46.7 ± 2.9AB | 86.0 ± 36.5A | 32.7 ± 6.2B | 38.0 ± 11.0B | 47.0 ± 3.6AB | 35.3 ± 5.4B | 8.3 ± 0.7C |
| totPLFA | 616 ± 78A | 601 ± 99A | 466 ± 114AB | 486 ± 76AB | 519 ± 4AB | 394 ± 88B | 121 ± 15C |
Mean values ± standard error for three different peat samples (Bliksrud 3, Killingmo 2, and Komnes 2). Different capital letters indicate significant differences within rows (ANOVA, P < 0.05).
Incubation at 25, 35, 45, and 55°C for 4 days.
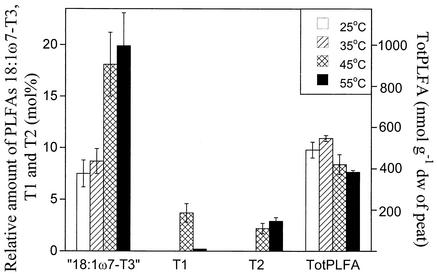
Heating of the peat to 55°C caused a significant reduction (P < 0.05) in totPLFA compared to the 25°C treatment, while the 35°C treatment did not differ from the 25°C control (Fig. 2). A PCA showed that differences in the PLFA pattern between peat samples were found in the first component (38% of the variation accounted for) while the second component (22% of the variation) differentiated between the different temperature treatments (data not shown). The loading values for the second component showed that the separation of the high-temperature treatments was mainly due to an increase in the amounts of T1, T2, and 18:1ω7-T3. The increases in the amounts of the last two PLFAs were closely correlated. The relative concentrations of T2 and 18:1ω7-T3 were highest in the 55°C treatment, while T1 had the highest relative concentration in the 45°C treatment (Fig. 2). T1 and T2 were not detected in the peat incubated at 35°C, and 18:1ω7-T3 had not increased in this treatment compared to the 25°C control.
FIG. 2.
totPLFA (millimoles per gram [dry weight] of peat) and moles percent of the PLFAs T1, T2, and 18:1ω7-T3 in peat samples incubated for 4 days at 25, 35, 45, or 55°C. Results are the mean of three peat samples (Bliksrud 3, Killingmo 2, and Komnes 2).
Short-term incubation at 55°C.
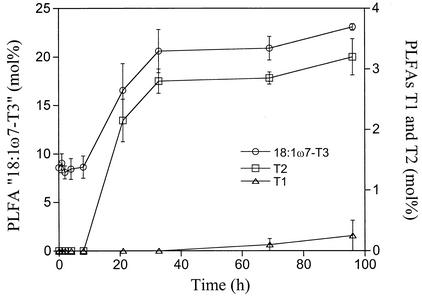
During the 96-h incubation at 55°C, the amount of totPLFA decreased from around 600 to around 450 nmol g−1 (dry weight) of peat (data not shown). Therefore, the amounts of most of the PLFAs decreased during this time. However, after about 4 to 8 h, the relative concentrations of the PLFAs 18:1ω7-T3 and T2 started to increase (Fig. 3); they stabilized after approximately 40 h. T1 was detected only in the two last samplings and thus had a different time course (Fig. 3).
FIG. 3.
Moles percent of the PLFAs T1, T2, and 18:1ω7-T3 in peat samples incubated at 55°C. Results are the mean values of two peat samples (Killingmo 2 and Komnes 2). Standard errors are indicated by bars.
Long-term incubation at 55°C and reestablishment of mesophilic microorganisms after heating to 55°C.
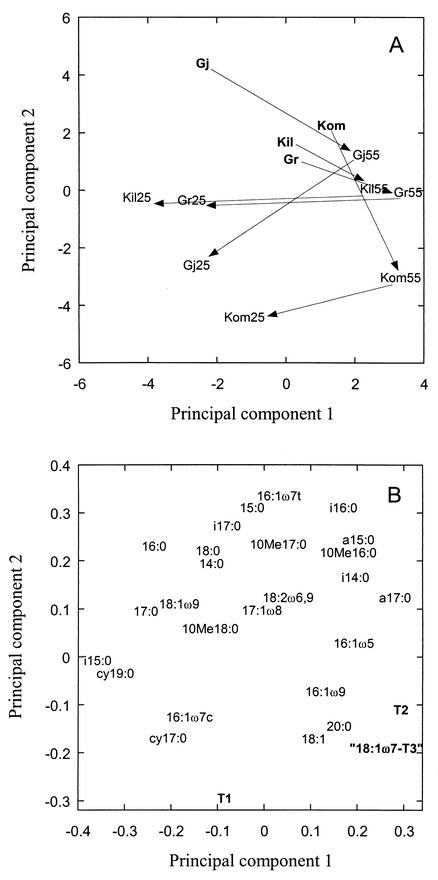
Peat samples were incubated at 55°C for 3 days and then at 25°C for up to 80 days. A PCA of the data showed that the changes in the PLFA pattern due to temperature alterations were similar in the different peat samples (Fig. 4A). The first principal component accounted for 26% of the variation in the data, and the second component accounted for 21%. The initial 55°C temperature treatment shifted the peat samples to the right in the PCA plot, while the subsequent incubation at 25°C shifted them to the lower left (indicated by arrows). The 55°C treatment was characterized by relatively large amounts of T2 and 18:1ω7-T3 (Fig. 4B). During the 25°C incubation, the amounts of these PLFAs decreased and those of i15:0, 16:1ω7c, cy17:0, and cy19:0 increased (Fig. 4B). However, the effect of the initial 55°C temperature treatment for 3 days was still evident, since the PLFA pattern was not restored even after 80 days at 25°C.
FIG. 4.
PCA of the PLFA pattern of peat samples incubated at different temperatures. Gj, Gjødalsmosan; Kil, Killingmo; Kom, Komnes; Gr, Greninmosan. Bold abbrevations indicate peat incubated at 25°C for 3 days, 55 indicates peat incubated at 55°C for 3 days, and 25 indicates peat incubated at 55°C for 3 days followed by incubation at 25°C for 80 days (Gj 1 and Kil 2), 30 days (Kom 2), or 50 days (Gr 3). (A) Score plot of the different peat samples. (B) Loading plot of the individual PLFAs. The PLFAs T1, T2, and 18:1ω7-T3 are in bold.
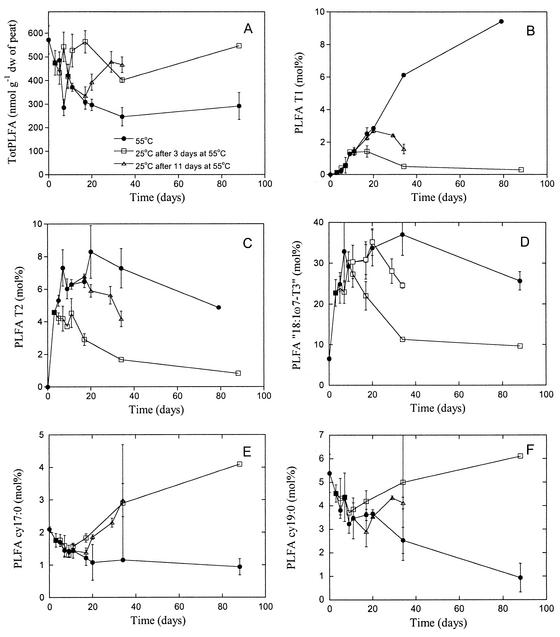
totPLFA decreased during the 80 days incubation at 55°C, from about 600 to around 300 nmol g−1 (dry weight) (Fig. 5A). When the peat incubation temperature was shifted from 55 to 25°C, totPLFA started to increase, irrespective of whether the temperature shift was made after 3 or 11 days at 55°C.
FIG. 5.
Development of PLFAs in peat samples incubated at 55°C (solid circles) followed by incubation at 25°C after 3 (open squares) or 11 (open triangles) days at 55°C. The value at time zero is the initial value before incubation at 55°C. (A) totPLFA; (B) T1, (C) T2; D) 18:1ω7-T3; (E) cy17:0; (F) cy19:0. Results are the means of three peat samples (Gjødalsmosan 1, Killingmo 2, and Komnes 2), except for the last sampling occasion at 55°C (only Gjødalsmosan 1 and Killingmo 2) and at 25°C (only Gjødalsmosan 1). Standard errors are indicated by bars.
The relative concentration of the PLFA T1 increased with incubation time at 55°C, until T1 eventually became one of the dominant PLFAs (about 10 mol% of totPLFA) after 80 days (Fig. 5B). This was also found in a separate experiment, where the amount of T1 increased to 9.5% ± 0.1% (n = 2 peat samples) after 2 months of incubation at 55°C. The amount of T2 increased rapidly during the first 3 days and stabilized at 5 to 8% of totPLFA throughout the study period (Fig. 5C). The amount of 18:1ω7-T3 increased from 7 to over 30% by day 10 and remained stable at this level (Fig. 5D).
T1 and T2 were affected differently by the temperature shift to 25 after the 55°C incubation. The relative concentration of T2 started to decrease a few days after the transfer, after both 3 and 11 days at 55°C, eventually ending up at around 1% of totPLFA after 80 days of incubation at 25°C (Fig. 5C). The relative concentration of T1 still increased for several days after the temperature shift, but eventually the amount of T1 started to decrease (Fig. 5B). However, after about 80 days at 25°C, both T1 and T2 were detectable. The amount of PLFA 18:1ω7-T3 started to decrease when the temperature was lowered from 55 to 25°C (Fig. 5D), and after 80 days similar levels to those before the 55°C incubation treatment were found.
Two PLFAs, cy17:0 and cy19:0, showed different responses to temperature compared to T1, T2, and 18:1ω7-T3. The relative concentrations of these PLFAs decreased slowly in peat samples incubated at 55°C (Fig. 5E and F), but after the temperature shift to 25°C, both started to increase after a few days. In particular, cy17:0 became relatively more abundant than at the start of the experiment.
Effect of different incubation temperatures after a heating event on the stability of PLFAs.
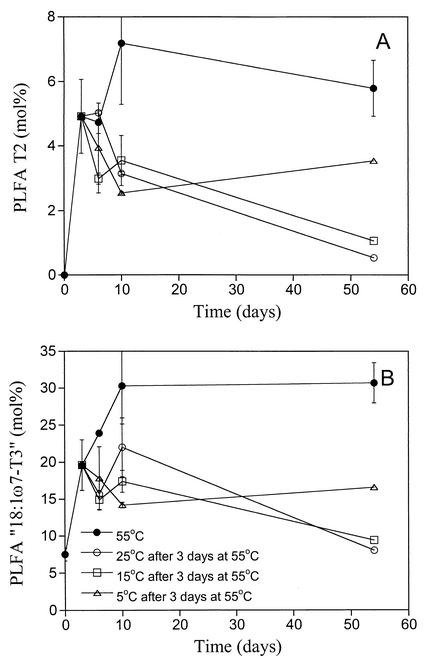
To simulate the effect of different temperatures during storage of peat after a heating event, samples were incubated for 3 days at 55°C and then transferred to different temperatures (5, 15, and 25°C). The amounts of the PLFAs T2 (Fig. 6A) and 18:1ω7-T3 (Fig. 6B) increased rapidly to around 6 and 30 mol% (of totPLFA), respectively, in the samples incubated at 55°C and then remained approximately stable for 40 days. After incubation at lower temperatures, the amounts of both these PLFAs decreased. This decrease appeared most evident at 25°C, while the decrease at 5°C was much smaller. After 2 months at 25°C, the amount of T2 constituted less than 1 mol% of the totPLFAs (similar to the long-term experiment above), while around 4 mol% was found in the peat samples incubated at 5°C (Fig. 6A). Peat samples incubated at 15°C had intermediate concentrations of T2. For 18:1ω7-T3, similar results were found (Fig. 6B).
FIG. 6.
The development of PLFAs (moles percent) in peat samples incubated at 55°C (solid circles), followed by incubation at 5°C (triangles), 15°C (squares), or 25°C (open circles) after 3 days at 55°C. (A) T2; (B) 18:1ω7-T3. Results are the, mean of two peat samples (Greninmosan 2 and Killingmo 2), except for the last sampling for the lower temperatures. Standard errors are indicated by bars.
The PLFAs cy17:0 and cy19:0 were affected differently from T1 and T2 (data not shown). The 55°C treatment decreased the amounts of both these PLFAs, and the shift to lower temperatures made them increase. This increase was most evident at 15 and 25°C, while hardly any increase was found at 5°C, even after 2 months incubation.
Pure-culture studies.
The PLFA patterns of the six bacterial isolates grown on peat agar at 55°C were similar. The most common PLFA was T3, which amounted to more than half of totPLFA. T2 also had high relative concentrations, amounting to about 10% of totPLFA. No other PLFAs, except the ubiquitous 16:0 and 18:0, were detected.
The PLFA T3 was the most common PLFA for isolates grown on tryptic soy agar at 55°C, with the amount of T2 being about 10% of the amount of T3. PLFAs 16:0 and 18:0 were also present. On TSA, several branched PLFAs, like i15:0 and i17:0, were present in amounts equal to that of T2. There were also traces of a15:0, i16:0, and i17:0. Incubation at 45°C gave a similar PLFA pattern to that at 55°C. T1 was never found. No growth was detected at 25°C.
GC-MS.
The methyl ester of the PLFA T1 had a relative retention time of 1.022 compared to that of the standard 19:0. The relative retention times for T2 and T3 were 1.067 and 0.912 (coeluting with 18:1ω7), respectively. The mass spectrum of the methyl ester of T1 (measured on one heated peat sample) was characterized by major peaks at 55, 74, 97, 143, 199, 253, and 296, the last being the molecular weight (MW) of the methylated fatty acid. The mass spectra of the methyl ester of T2 (measured on two pure culture isolates and one heated peat sample) had major peaks at 55, 74, 83, 143, 199, 267, and 310 (the MW). The methyl ester of T3 (measured on two pure culture isolates) was characterized by major peaks at 55, 74, 83, 143, 199, 239, and 282. The MW, 282, was clearly different from that of 18:1ω7 (MW, 296). In a heated peat sample, the 18:1ω7-T3 peak had a mass spectrum with peaks at both 282 and 296, indicating that we could not separate these two PLFAs in peat samples with the gas chromatography (GC) setup used.
The lack of silver adducts showed that T1, T2, and T3 were saturated fatty acid methyl esters. They had 19, 20, and 18 carbons atoms, respectively, as indicated by high-resolution mass spectrometry (MS). The long GC retention times indicated a cyclic ring structure at the terminal end of the fatty acyl chain. The increased abundance, compared to normal acyl chains, of m/z 83, as well as 227 (M − 83; T2) and 199 (T3), indicated a terminal saturated hexyl ring. A standard preparation of fatty acids from Alicyclobacillus (courtesy of Karl Poralla, Tübingen University, Tübingen, Germany) showed that methyl ω-cyclohexyltridecanoate eluated with the same retention time as T2 and methyl ω-cyclohexylundecanoate eluted with the same retention time as T3. No standard was compared with T1. However, the presence of relatively high levels of m/z 97 and 199 (M − 97) suggest that T1 could be methyl ω-cycloheptylundeconate. The mass spectrum was similar to those published for this compound (19).
DISCUSSION
The PLFAs T1 and T2 appeared to be good indicators of a previous period of high temperature in peat. They were never detected in unheated peat in our study, and PLFAs with similar retention times in the GC analyses were not found in natural peat samples (3, 23). These PLFAs have not been observed in a variety of soils (1, 2, 8), and they were not reported from different composts (6, 16).
The PLFAs T1 and T2 are most probably produced by two different organisms, since they did not have the same development pattern, with respect to both the effect of different incubation temperature on them (Fig. 2) and their growth pattern over time (Fig. 5B and C). T1 is probably produced by an organism with a lower temperature optimum for growth, since it was produced faster at 45 than at 55°C, while the opposite was the case for T2 (Fig. 2). The organism producing T1 appeared to be able to grow slowly at 55°C (Fig. 5B) and could probably compete well with other organisms, since T1 became a dominant PLFA after prolonged incubations at 55°C.
The identification of T1 as an ω-cycloheptyl and T2 and T3 as ω-cyclohexyl fatty acids indicated that the microorganisms were members of the genus Alicyclobacillus, which is characterized by large amounts of these rare fatty acids (4, 10, 19). Among Bacillus-related genera, Alicyclobacillus is characterized as thermophilic, aerobic, heterotrophic, and preferring acid conditions (4, 5), which fits with the environmental conditions in heated peat.
Besides the production of specific PLFAs during heating, the turnover of these PLFAs under the following mesophilic conditions is of utmost importance if they are to be used as indicators of an earlier heating event. It is assumed that the decomposition of PLFAs is fast once the organisms die (15, 25). The initial decomposition of phospholipids involves the removal of the phosphate group by phosphatases. This alters the polar phospholipid into a neutral lipid, which is not included in the analysis. When mesophilic microorganisms were killed by heating in a compost, a fast turnover was found during the heating phase, with half-lives of some PLFAs of 1 day (16). A high temperature kills the mesophilic organisms, and the activity of enzymes decomposing the phospholipids is high. The situation is different when a temperature lower than the temperature permissible for growth is studied. A low temperature does not directly kill the organisms but just renders them inactive. Furthermore, once they have died, decomposition is slow due to low temperatures making enzymatic reactions proceed more slowly. This appeared to be the case in the compost study (16), where the PLFA i17:0 produced by thermophilic organisms had a half-life of 1 week when the temperature decreased to 30°C. In a recent study (6), PLFAs produced during the thermophilic phase could even be detected in 1-year-old composts.
In the present study, the amount of T2 decreased slowly when the peat samples were incubated at 25°C after 3 or 11 days at 55°C (Fig. 5C). Even after 3 months at 25°C, T2 was still detected, indicating a slow turnover. Similar results were found for T1 (Fig. 5B). In this case, the relative concentration of the PLFA did not decrease until after about 2 weeks, indicating that growth of the organism responsible for producing T1 was possible at 25°C.
When the temperature is lowered, the two processes (inactivation-death and decomposition) responsible for the disappearance of the PLFAs will have different influences on the PLFAs from thermophilic organisms. The lower the temperature, the more likely it is that a thermophilic organism will become dormant or die, since the selection pressure against thermophiles would increase as the temperature decreases. Thus, one would expect PLFAs from thermophilic organisms to disappear faster at lower temperatures than at temperatures close to the minimum for growth. This was the case with the thermophilic bacterial activity (measured as thymidine incorporation [21]). When peat samples were incubated at 55°C to favor thermophilic microorganisms and then shifted to 25, 15, or 5°C, the thermophilic bacterial activity disappeared immediately at 5°C, within 7 days at 15°C, and after 1 month at 25°C. The second process (the decomposition of the PLFAs), on the other hand, proceeds more slowly as the temperature is reduced. The decomposition of PLFAs indicative of thermophilic organisms appeared to be the most important factor, since both T2 and 18:1ω7-T3 disappeared faster at 25 than at 5°C (Fig. 6). Therefore, the faster the temperature decreases and the lower it stays after a heating event, the more likely it is that T2 and T3 will be detected after a long storage period.
When mesophilic conditions (25°C) were restored after incubation at 55°C, mesophilic organisms started to grow and the amounts of PLFAs from these organisms increased. This was, for example, the case with cy17:0 and cy19:0 (Fig. 5E and F). Cyclopropane fatty acids are common in gram-negative bacteria (26), indicating that the proportion of gram-negative bacteria increased during the mesophilic conditions. This is also commonly found in composts after the heating phase (12, 16, 17). The increase in the numbers of gram-negative bacteria after the heating event is probably not only due to the mesophilic temperature conditions per se but also due to fresh nutrients being released by the heat treatment (see Table 1). Gram-negative bacteria are known to thrive under conditions of ample nutrient supply, with high microbial activity, for example, occurring in the rhizosphere (14).
Low temperatures appeared to both preserve PLFAs characteristic for the thermophiles (T2 and 18:1ω7-T3 [Fig 6]), and slow the recovery of PLFAs indicative of mesophiles (cy17:0 and cy19:0). The former result has been discussed above. The reason for the latter is that mesophilic organisms have optimum temperatures for growth at 25 to 30°C. The recovery would be faster at temperatures around the optimum temperatures for growth than at lower temperatures. To be able to interpret the temporal changes in PLFA pattern under different temperature regimes, one has to take into account the fact that the effect of temperature on death and growth rate, as well as on the decomposition rate of PLFAs from dead organisms, is different for mesophilic and thermophilic organisms. Therefore, an interpretation of the temporal changes in PLFA pattern is difficult. The use of techniques that measure direct activity, like the thymidine incorporation technique (21), should be helpful in such interpretations, since these techniques are not dependent on the decomposition of a specific substance to measure a decrease in the amount of an organism group.
The production of T2 and T3 (the latter measured as an increase in the amount of 18:1ω7-T3) was fast in heated peat, and maximum levels were found within 3 days at 55°C, when little or no T1 was detected (Fig. 2 and 3). In the self-heated peat, the relative concentration of T2 was mostly higher than the concentration of T1; only once was T1 detected in a sample where no T2 was found. This may indicate that the shift in temperature in the stockpiles during self-heating was fast, not permitting T1 to form in detectable amounts, or that the temperature became too high for the organism producing T1. This suggests that T2 or T3 might be the best PLFAs to use as characteristic for heated peat.
To use the PLFAs T1, T2, and T3 as indicators of previous self-heated peat, it will be important to determine whether the production of these PLFAs can be correlated with the production of compounds toxic to plant growth. If this is the case, it would be possible to use the presence of T2 and T3 to detect such peat samples. However, as stated above, the conditions after the heating event will affect the amounts of these PLFAs detected.
Acknowledgments
This study was supported by grants to E.B. from the Swedish Natural Science Research Council. S.B.R. was financially supported by Jiffy Pro. Ltd., NGF, and the Norwegian Research Council (project 103.195).
We thank J. Vedde and E. Uggerud at Oslo University, Oslo, Norway, and A. Smith at the Macaulay Land Use Research Institute, Aberdeen, United Kingdom, for help with the GC-MS analyses and K. Poralla, Tübingen University, Tübingen, Germany, for the gift of the fatty acid standards of Alicyclobacillus.
REFERENCES
- 1.Bååth, E., Å. Frostegård, and H. Fritze. 1992. Soil bacterial biomass, activity, phospholipid fatty acid pattern, and pH tolerance in an area polluted with alkaline dust deposition. Appl. Environ. Microbiol. 58:4026-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bååth, E., Å. Frostegård, T. Pennanen, and H. Fritze. 1995. Microbial community structure and pH response in relation to soil organic matter quality in wood ash fertilized, clear cut or burned coniferous forest soils. Soil Biol. Biochem. 27:229-240. [Google Scholar]
- 3.Borgå, P., M. Nilsson, and A. Tunlid. 1994. Bacterial communities in peat in relation to botanical composition as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 26:841-848. [Google Scholar]
- 4.Deinhard, G., P. Blanz, K. Poralla, and E. Altan. 1987. Bacillus acidoterrestris sp. nov., a new thermotolerant acidophile isolated from different soils. Syst. Appl. Microbiol. 10:47-53. [Google Scholar]
- 5.Deinhard, G., J. Saar, W. Krischke, and K. Poralla. 1987. Bacillus cycloheptanicus sp. nov., a new thermoacidophile containing ω-cycloheptane fatty acids. Syst. Appl. Microbiol. 10:68-73. [Google Scholar]
- 6.Eiland, F., M. Klamer, A.-M. Lind, M. Leth, and E. Bååth. 2001. Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb. Ecol. 41:272-280. [DOI] [PubMed] [Google Scholar]
- 7.Finstein, M. S., and M. L. Morris. 1975. Microbiology of municipal solid waste composting. Adv. Appl. Microbiol. 19:113-151. [DOI] [PubMed] [Google Scholar]
- 8.Frostegård, Å., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gärdenäs, S., and T. Thörnqvist. 1984. Spontaneous combustion and dry matter losses in peat storage. Report 156. Department of Forest Products, The Swedish University of Agricultural Sciences, Uppsala.
- 10.Goto, K., H. Matsubara, K. Mochida, T. Matsumara, Y. Hara, M. Niwa, and K. Yamasato. 2002. Alicyclobacillus herbarius sp. nov., a novel bacterium containing ω-cycloheptane fatty acids, isolated from herbal tea. Int. J. Syst. E vol. Microbiol. 52:109-113. [DOI] [PubMed] [Google Scholar]
- 11.Hellmann, B., L. Zelles, A. Palojärvi, and Q. Bai. 1997. Emission of climate-relevant trace gases and succession of microbial communities during open-window composting. Appl. Environ. Microbiol. 63:1011-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann, R. F., and J. F. Shann. 1997. Microbial community changes during composting of municipal solid waste. Microb. Ecol. 33:78-85. [DOI] [PubMed] [Google Scholar]
- 13.Hodnebrog, T., and A. R. Selmer-Olsen. 1998. Mottakskontroll for veksttorv. Fagnytt no. 1. Department of Horticulture and Crop Sciences, Agricultural University of Norway, Ås.
- 14.Ibekwe, A. M., and A. C. Kennedy. 1999. Fatty acid methyl ester (FAME) profiles as a tool to investigate community structure of two agricultural soils. Plant Soil 206:151-161. [Google Scholar]
- 15.King, J. D., D. C. White, and C. W. Taylor. 1977. Use of lipid composition and metabolism to examine structure and activity of estuarine detrital microflora. Appl. Environ. Microbiol. 33:1177-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klamer, M., and E. Bååth. 1998. Microbial community dynamics during composting of straw material studied using phospholipid fatty acid analysis. FEMS Microbiol. Ecol. 27:9-20. [Google Scholar]
- 17.Klamer, M., F. Eiland, A.-M. Lind, M. Leth, J. J. L. Iversen, U. Söchting, and E. Bååth. 2000. Changes in chemical composition and microbial biomass during composting of straw and pig slurry, p. 322-334. In P. R. Warman and B. R. Taylor, (ed.), Proceedings of the International Composting Symposium (ICS'99). CBA Press, New York, N.Y.
- 18.McKinley, V. L., and J. R. Vestal. 1984. Biokinetic analyses of adaptation and succession: microbial activity in composting municipal sewage sludge. Appl. Environ. Microbiol. 47:933-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, B. S., H. G. Floss, and K. Poralla. 1995. Three new ω-cycloheptyl fatty acids from Alicyclobacillus cycloheptanicus and their biosynthetic interrelationships. J. Nat. Prod. 58:590-593. [DOI] [PubMed] [Google Scholar]
- 20.Puustjärvi, V. 1983. Effect of self-heating in stockpiles on the structure of horticultural peat, p. 57-63. In Peat and plant yearbook. Association of Finish Peat Industries, Helsinki, Finland.
- 21.Ranneklev, S. B., and E. Bååth. 2001. Temperature-driven adaptation of the bacterial community in peat measured by using thymidine and leucine incorporation. Appl. Environ. Microbiol. 67:1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strom, P. F. 1985. Effect of temperature on bacterial species diversity in thermophilic solid-waste composting. Appl. Environ. Microbiol. 50:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundh, I., M. Nilsson, and P. Borgå. 1997. Variation in microbial community structure in two boreal peatlands as determined by analysis of phospholipid fatty acid profiles. Appl. Environ. Microbiol. 63:1476-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wever, G., and M. H. Hertogh-Pon. 1993. Effects of self-heating on biological, chemical and physical characteristics of peat. Acta Horticult. 342:15-24. [Google Scholar]
- 25.White, D. C., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1978. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 26.Zelles, L. 1997. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275-294. [DOI] [PubMed] [Google Scholar]