Abstract
Pigment-dispersing factor (PDF) is a neuropeptide that plays a prominent role in the circadian clock of several insects. The cockroach Leucophaea maderae was the first animal where the site of a biological clock could be located, and still is a focal point of circadian research. Although detailed studies on the action of pigment-dispersing factor and the distribution of PDF-like immunoreactivity in the L. maderae brain exist, a native pigment-dispersing factor of this species has not been characterized so far. The authentic Lem-PDF was isolated from L. maderae by a combination of high performance liquid chromatography, crab pigment-dispersion bioassay and an immunosorbent assay. Mass spectrometric characterization and the conserved sequence of pigment-dispersing factor in orthopteromorphan insects suggest that Lem-PDF has the sequence NSEXINSLLGLPKVLNDAa (where X= I or L). Lem-PDF is thus identical to either Periplaneta americana PDF or Acheta domesticus PDF. Detailed analysis of PDF-like immunofluorescence in different regions of the brain suggests that there are no drastic daily changes in the amount of pigment-dispersing factor as occur in Drosophila melanogaster, which might be explained by a lack of circadian pigment-dispersing factor release and production, or by phase differences between the pigment-dispersing factor neurons.
Abbreviations
ESI-Q-TOF MS electrospray ionization-quadrupole-time of flight mass spectrometry
MALDI-TOF MS matrix-assisted laser desorption ionization-time of flight mass spectrometry
PDF pigment-dispersing factor (Drm-PDF Drosophila melanogaster-PDF; Lem-PDF, Leucophaea maderae-PDF; Pea-PDF, Periplaneta americana-PDF)
PDH pigment-dispersing hormone
s-LNvs and lLNvs small and large ventral lateral neurons
ZT Zeitgeber time
Keywords: neuropeptides, circadian clock, insect brain, MALDI-TOF MS
Introduction
Insect pigment-dispersing factors are neuropeptides with a highly conserved 18 amino acid sequence, and are homologues to the pigment-dispersing hormones (PDH) of crustaceans (Rao and Riehm, 1988). To date, pigment-dispersing factors have been identified in several dipteran insect species as well as in Orthopteromorpha and Hemiptera (Rao et al., 1987; Rao and Riehm, 1988; Mohrherr et al., 1991, 1994; Nässel et al., 1993; Park and Hall, 1998; Sato et al., 2002; Chuman et al., 2002; Singaravel et al., 2003; Matsushima et al., 2004a, b).
It is clear that pigment-dispersing factors play a prominent role in the circadian system in several insect species. In the fruit fly Drosophila melanogaster, there is good evidence that pigment-dispersing factor is a major output factor from the main pacemaker neurons that comprise the circadian clock (Renn et al., 1999; Park et al., 2000). The cockroach, Leucophaea maderae, has been used extensively as a model insect in circadian clock research (see Homberg et al., 2003). In fact, L. maderae was the first animal where a circadian pacemaker could be localized to a distinct brain area, the optic lobe (Nishiitsutsuji-Uwo and Pittendrigh, 1968). It was also in L. maderae and other Orthopteromorpha that a role for pigment-dispersing factor within the circadian clock was first proposed when it was found that some PDH-like immunoreactive neurons fulfil several anatomical criteria proposed for circadian pacemakers in the brain (Homberg et al., 1991). Surgical lesion studies in L. maderae led to the suggestion that pigment-dispersing factor acts as an output signal for circadian activity rhythms (Stengl and Homberg, 1994; Reischig and Stengl, 2003, Reischig, 2003). In addition, pharmacological and morphological studies suggest that pigment-dispersing factor conveys a coupling signal between the different pacemaker neurons, and further support a role for it as a circadian output factor for the locomotor activity rhythm (Petri and Stengl, 1997; Reischig and Stengl, 2002; Reischig et al., 2004). However, an authentic pigment-dispersing factor has not yet been characterized in L. maderae.
In adult D. melanogaster, pigment-dispersing factor is localized in circadian pacemaker neurons known as the small and large ventral lateral neurons (s-LNvs and l-LNvs respectively) (see Helfrich-Förster, 2003). A null mutation in pdf as well as ectopic expression of pigment-dispersing factor in the dorsal protocerebrum disrupts normal circadian locomotor rhythms (Renn et al., 1999; Helfrich-Förster et al., 2000). Pigment-dispersing factor immunostaining in the terminals of the sLNvs in the dorsal protocerebrum but not within the somata exhibits a daily and circadian cycle with a peak during early morning, which apparently corresponds to the daily locomotor activity of D. melanogaster (Park et al., 2000). Since the amount of pdf mRNA does not exhibit daily cycles (Park et al., 2000; Matsushima et al., 2004b), it is likely that the release of pigment-dispersing factor from the dorsal terminals of the s-LNvs is regulated in a circadian fashion.
In this study, we analyzed changes in pigment-dispersing factor-like immunoreactivity at different time points throughout the day in different portions of the brain of L. maderae by means of immunofluorescence staining. In addition, we isolated pigment-dispersing factor from the brain of L. maderae and characterized its sequence by mass spectrometry.
Our results show that L. maderae possesses a native orthopteroid-type pigment-dispersing factor, and suggest that the amount of pigment-dispersing factor in the cell bodies and terminals of the pacemaker neurons of L. maderae does not change significantly over the light-dark cycle.
Materials and Methods
Animals
Cockroaches, L. maderae, were kept at about 26° C under a 12:12 hours LD regime and were fed dog food pellets, vegetables and water ad libitum. For analysis of PDF-like immunoreactivity by immunostaining, we used only male cockroaches 2 to 5 weeks after adult ecdysis.
Fruit flies, D. melanogaster, were kept at 18° C on standard food at LD 12:12. The pdf01 strain was a kind gift of Paul Taghert (St. Louis, MO).
Tissue extraction and prepurification of pigment-dispersing factor
A total of 1000 L. maderae brains were dissected under ice-cold cockroach saline or phosphate buffered saline (0.1M, pH 7.4). Batches of approximately 200 brains were collected into an Eppendorf tube kept at −20° C in a labtop cooler and were stored at −70° C until further processing. Each batch was homogenized with a teflon pestle in 1 ml of ice-cold extraction medium (90% methanol, 9% H2O and 1% trifluoroacetic acid (TFA), (v/v/v). This extraction medium was found to be more effective than 50% methanol and 0.1% acetic acid. After centrifugation at 9800 rpm for 10 minutes at 4° C, the supernatant was collected as extract. The pellet was re-suspended in 1 ml extraction medium and homogenized and extracted as described. The two supernatants were pooled, dried in a vacuum centrifuge (Savant, Speed-Vac Concentrator, www.savec.com), and kept in a desiccator at room temperature in the dark until further processing. The dried extracts were dissolved in 1ml 0.1% TFA solution and sonicated. An equivalent of 500 brains was loaded onto a Sep-Pack C18 cartridge (20 ml; Waters, www.waters.com), which was previously activated with 50 ml acetonitrile (AcN), washed with 50 ml H2O and equilibrated with 50 ml 0.1%TFA solution. The cartridge was then washed with 15 ml 10% AcN containing 0.1%TFA, and peptides were eluted with 4 ml 60% AcN containing 0.1%TFA.
Reverse-phase HPLC
Separations were carried out with a high performance liquid chromatography (HPLC) system (Waters) consisting of a 600E system controller and a 486 ultraviolet (UV) detector set to 214 nm.
For the separation of the 2 × 500 brain equivalents during the isolation of Lem-PDF, the prepurified sample was diluted with 0.1% TFA solution until the solvent corresponded to 15% AcN, and directly pumped onto a semipreparative diphenyl column (Vydac 219TP54 5µm, 4.6 mm × 250 mm, www.vydac.com). After loading, 15% AcN with 0.1% TFA was pumped for 12 min at isocratic condition (i.e. with a constant mobile phase composition). Peptides were eluted by a linear gradient to 45% AcN containing 0.1% TFA during 60 min at 1ml/min flow rate, and fractions were collected each minute. All fractions were checked for PDF-immunoreactivity (by ELISA) and PDF-bioactivity (by a pigment-dispersion bioassay in a pre-run).
Only the fractions that showed both immunoreactivity and bioactivity were resolved with 5 ml of 15% AcN containing 0.1% TFA, pooled and loaded onto an analytical C18 column (Vydac, 4.5µm, 4.6 × 150 mm). After 3 min isocratic condition with 15% AcN containing 0.1% TFA, the sample was eluted by a linear gradient to 45% AcN containing 0.1% TFA at 1ml/min flow rate. The prominent peaks were collected manually and vacuum-dried.
For the third separation, 0.1% heptafluorobutyric acid was added to the mobile phase as ion pairing reagent. Samples were eluted with a linear gradient from 15% AcN to 30% AcN for 10 min followed by a linear gradient to 60% AcN for 45 min at 1ml/min flow rate. The prominent peaks were collected manually.
For the last separation, the sample was eluted from an analytical phenyl-hexyl column (Luna, 5µm 2.0 × 150 mm; Phenomenex, www.phenomenex.com) with a linear gradient of 20% AcN with 0.1%TFA to 70% AcN with 0.1%TFA at 0.2ml/min. The prominent peaks were collected manually.
From each fraction collected during the different separation steps, an equivalent of 10 or 30 brains were measured by matrix-assisted laser desorption ionization - time of flight mass spectrometry (MALDI-TOF MS) or ELISA, respectively. To exclude contamination by synthetic standards (Drm-PDF from D. melanogaster or Pea-PDF from Periplaneta americana), a test run using 15% or 20% AcN containing 0.1% TFA as sample was performed prior to each separation during the isolation procedure. Fractions were collected each minute and assayed in ELISA. In all these test runs, PDF-like immunoreactivity could not be detected in any fraction.
Enzyme-linked immunosorbent assay (ELISA)
PDF-immunoreactivity was assayed in an indirect competitive ELISA using antiserum raised against Drm- PDF (Persson et al., 2001). MaxiSorp F96 immunoplates (Nunc, www.nuncbrand.com) were coated with 5 ng/well of a human serum albumin-glutaraldehyde-Drm-PDF conjugate in 100 µl of 0.02 M PBS (pH 7.2) for 3 hours on a shaker. Each well was then washed with 300 µl wash buffer (0.02M Tris buffer containing 0.05% Tween and 0.1% gelatine, pH7.4), and blocked with 200 µl TBS-Tween containing 0.5% gelatine. After washing 3× with wash buffer, 50 µl of known amounts of Drm-PDF (in triplicate) or test samples (at least in duplicates) and 50 µl antiserum, diluted 1:80,000 in wash buffer, were added per well and incubated overnight at 4° C on a shaker. Then, plates were washed three times, and 100 µl of an alkaline-phosphatase conjugated goat-anti-rabbit IgG (Pierce, Rockford, IL) diluted 1:2,000 in wash buffer were added per well. After 2 h on a shaker, plates were again washed three times. The bound enzyme was detected by substrate reaction with p-nitrophenylphosphate (Merck, www.merck.com), 100 µl/well of a 1 mg/ml solution in 0.05 M diethanolamine buffer containing 0.05 M MgCl2 and 0.02% NaN3, pH 9.8 for 30-45 minutes. The enzyme reaction was stopped after 30 min with 100 µl 3 M NaOH and absorption was measured in an ELISA plate reader (Multiskan Plus, www.mtxlsi.com) at 405 nm. If not stated otherwise, all steps were carried out at room temperature. The 4-parameter curve-fitting of GraphPad Prism 3 was used to evaluate the results of the ELISA readings.
To test whether the antiserum raised against unoxidized Drm-PDF also recognizes Met15-oxidized Drm-PDF, 200 nmol synthetic Drm-PDF was incubated with 200 µl 0.1M H2O2 for 4 h at 37° C. This treatment resulted in near-to-complete oxidation of the Drm-PDF without detectable fragmentation as checked by reverse-phase HPLC and MALDI-TOF MS (data not shown).
Mass spectrometry
MALDI-TOF MS: 0.5 µl of α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich, www.sigmaaldrich.com) in 50% AcN containing 0.1% TFA was mixed with 0.5 µl of the HPLC fractions on a stainless steel target and left to dry (dried droplet method). Masses were recorded with an Applied Biosystems Voyager DE-STR MALDI-TOF mass spectrometer set to the positive reflector mode. Prior to analysis, the mass spectrometer was calibrated using external standards.
ESI-Q-TOF MS (electrospray ionization-quadrupole-time of flight mass spectrometry): Nanoelectrospray mass spectra were acquired in the positive-ion mode using the API Qstar Pulsar (Applied Biosystems, www.appliedbiosystems.com) fitted with a Protana (www.protana.com) nanoelectrospray source. An aliquot (2 µl) of the HPLC- fraction containing the putative PDF-peptide was loaded onto the source and analyzed. After determining the mass over charge (m/z) of the peptide in MS mode, a collision energy of 15-45 V was applied. The m/z of interest was isolated and fragmented with the instrument in “enhance all” mode. MS/MS data of double and triple charged ions were acquired over 5 minutes and manually analyzed.
Pigment-dispersion bioassay for isolation
An extract from 60 L. maderae brains was separated using the same semipreparative diphenyl column and conditions as described for the first separation step during isolation. Half of the amount of the collected minute-fractions was tested for bioactivity in the pigment-dispersion bioassay using destalked fiddler crabs, Uca pugilator, as described by Rao et al. (1987). The other half was tested for PDF-immunoreactivity in ELISA.
Immunocytochemistry for quantification of PDF-like material
The protocol for immunocytochemistry followed Nässel et al. (1993). L. maderae brains were dissected at Zeitgeber time (ZT) 0, 3, 6, 9, 12, 15, 18 and 21 ± 10 min. ZT 0 was defined at lights on (= beginning of the day) and ZT 12 as lights off (= beginning of the night). The animals from dark phase (ZT12-21) were dissected under weak red light. The dissected brains were immediately fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 6 hours and sectioned with a cryostat at 25 µm thickness. Anti-β-PDH antiserum (characterized by Dircksen et al., 1991; Nässel et al., 1993; Stengl and Homberg, 1994) was applied at a dilution of 1:10,000 over night. After rinsing with 0.05 M phosphate buffer saline containing Triton X-100 (PBS-Tx), sections were incubated with a Cy2-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, www.jacksonimmuno.com) at a dilution of 1:500 for 24 hours. After rinsing with PBS-Tx, sections were embedded with 80% glycerol in PBS.
The immunostained brain-sections were imaged with a motorized Zeiss Axioplan II microscope and a cooled CCD camera (Hamamatsu 4742-95, www.hamamatsu.com), both operated with OpenLab 3 software (Improvision, www.improvision.com). Exposure time and gain were fixed to values such that no pixel was saturated in a gray scale range. The optic lobe and the protocerebral area of the sections were separately imaged. The captured images were converted to TIFF files and transferred to NIH image (http://rsb.info.nih.gov/nih-image). Referring to a previous paper (Reischig and Stengl, 2002), brain regions containing the β-PDH-immunoreactive neurons were chosen (see below for the details) and staining intensity of each brain region was scored into a gray-scale unit between 0 (black) and 255 (white).
Since no staining had been detected in the antennal lobes by the β-PDH antiserum, we set the gray-value in the antennal lobe as background fluorescence. The pixels of the anti-PDH-immunoreactivity were identified by the gray-values higher than the highest gray-value of the antennal lobes in the same brain hemisphere of the same individual, and the background values were then subtracted. The resulting gray-values corresponding to β.PDH-reactivity were arranged into a histogram for each brain region of each section. Since some β-PDH-immunoreactive neurons lay across different slices of the sections, the histograms of those regions were assembled within the same brain hemisphere of the same individuals. The mean gray-value of the 10% pixels with the highest fluorescence in each brain region of each brain hemisphere was calculated for each individual as a staining index. The data were standardized to the staining index at ZT12.
Based on the location of the terminals and cell bodies (referring to Reischig and Stengl, 2002), the β-PDH-immunoreactive neurons were classified into three regions: “lamina”, “anterior medulla” and “posterior medulla”. Since the dorsal lamina cell bodies were sometimes broken during sectioning, we measured staining index only in the ventral side of the lamina cell bodies. The terminals of the pigment-dispersing factor neurons were classified into four brain regions; the anterior optic commissure, the superior lateral protocerebrum, the ventro-lateral protocerebrum, the posterior optic tract, and two optic lobe regions: the lamina and the anterior portion of the medulla. Data from brain regions containing a damaged section were discarded.
Results
Validation of the ELISA
Since the antiserum used for ELISA was produced against Drm-PDF, the assay was evaluated using synthetic pigment-dispersing factors of D. melanogaster and P. americana as well as HPLC-separated extracts of OrR wild-type (wt) or pdf01 null mutant (Renn et al., 1999) fruit flies. The EC50 (i.e. half the concentration of free peptide needed for maximal competition) values for the ELISA standard curves were 26 ± 40 nM (mean ± S.E.M.) for both M15 oxidized and reduced form of Drm-PDF (n = 37), and 191 ± 46 nM for Pea-PDF (n = 2, Fig. 1). As can be seen in Figure 2, three immunoreactive peaks were found in an extract of 200 whole heads of wt flies. Peak 1 (minute-fractions 33/34) corresponded to the retention time of M15 oxidized Drm-PDF, peak 2 (minute-fraction 37) corresponded to Drm-PDF, and peak 3 (minute-fraction 41) contains unknown immunoreactive material. Peaks 1 and 2 were not found in extracts of 200 whole heads of pdf01 null mutants. Peak 3 was still visible, indicating that the unknown immunoreactive material is not related to pigment-dispersing factor. Based on the ELISA data from peak 1 and 2, about 1.3 fmol pigment-dispersing factor are present in the head of one fly.
Figure 1.
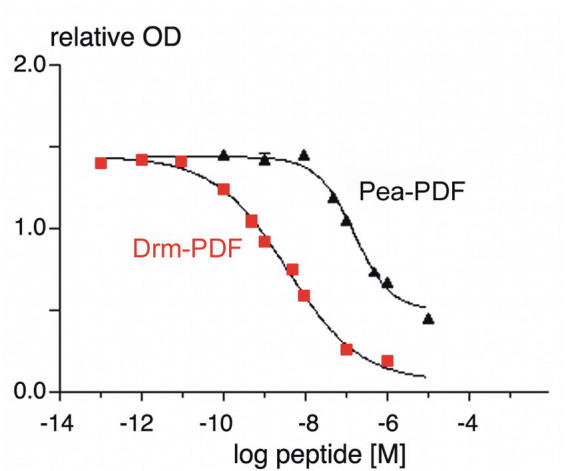
Dose-response curves of synthetic Drm-and Pea-PDF in the indirect competitive Drm-PDF ELISA. Absorption is given as relative optical density (OD) at 405 nm.
Figure 2.
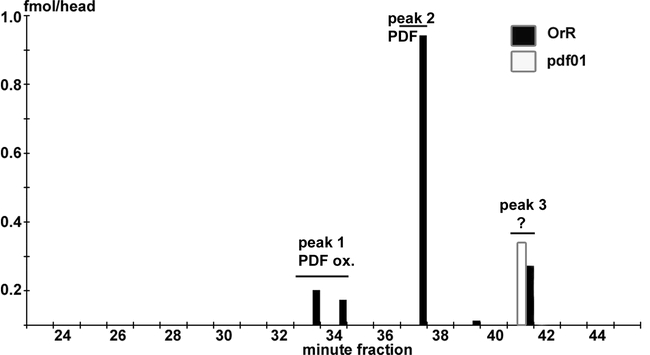
PDF-like immunoreactivity in fractions separated by HPLC from extracts of 200 whole heads of adult wildtype (OrR) and pdf null mutant (pdf01) Drosophila melanogaster. Peak 1 corresponds to M15 oxidized Drm-PDF, peak 2 to Drm-PDF, and peak 3 to an unknown substance. Unlike peak 3, peaks 1 and 2 were not present in pdf01 flies. Thus it is unlikely that the immunoreactivity in peak 3 represents a pdf precursor resulting from propeptide cleavage.
In cockroaches, the pattern of PDF-like immunoreactive material in HPLC-separated extracts was more complex than in the flies (see below, Fig. 3).
Figure 3.
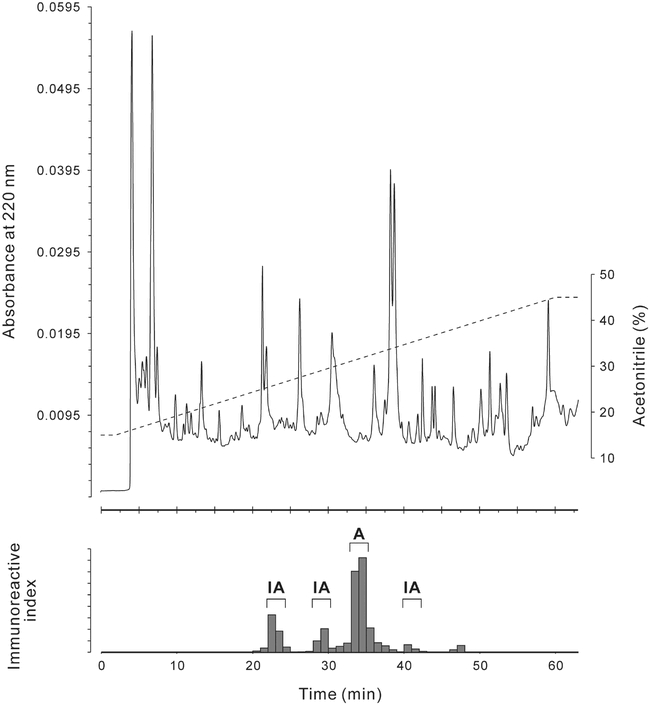
Top: HPLC chromatogram of an extract of 60 Leucophaea maderae brains separated on a diphenyl column under similar conditions as the first separation step described in Materials & Methods (../#materials). Bottom: Immunoreactivity of the minute-fractions separated above as measured with the anti-Drm-PDF antiserum. Only the largest immunoreactive peak revealed pigment-dispersing bioactivity (A), all others were inactive (IA).
Isolation and mass spectrometric characterization of Lem-PDF
Prior to the isolation, an extract of 60 brains with optic lobes was prepared and separated in a pre-run under conditions identical to those used for the first HPLC separation step of the isolation. An aliquot equivalent to 25.7 brains of the collected 1-min-fractions was tested for immunoreactivity using the ELISA. One prominent and three small immunoreactive peaks were found (Fig. 3). The remaining 34.3 brain equivalents of the immunoreactive minute-fractions were pooled for each immunoreactive peak and tested for pigment-dispersion activity in destalked fiddler crabs, Uca pugilator. Out of the four analysed peaks, only the pooled immunoreactive 1-min-fractions 34-35; i.e. those with the highest immunoreactivity, showed significant melanophore dispersion activity with a standard integrative response value (Fingerman et al., 1967) of 3.6, all others were inactive (Fig. 3). Based on the ELISA, the total amount of PDF-like bio- and immunoreactive material was i) 150 fmol/brain, ii) 80 fmol/brain without optic lobes, and iii) 26 fmol/optic lobe.
For the first HPLC separation step, each batch containing 500 brains was chromatographed separately, and the collected 1-min-fractions were measured in ELISA. The main peak of PDF-immunoreactivity (around 2.3 pmol/brain) was found in fractions 36-38. Since the fractions of the pre-run that contained the highest immunoreactivity had a similar retention time and showed bioactivity, it was concluded that fractions 36-38 should contain the bioactive material. MALDI-TOF analysis of fractions 36-38 revealed a multitude of mass peaks, but in fraction 37 a peak with a mass identical to Pea-PDF and Acd-PDF was found (Fig. 4).
Figure 4.
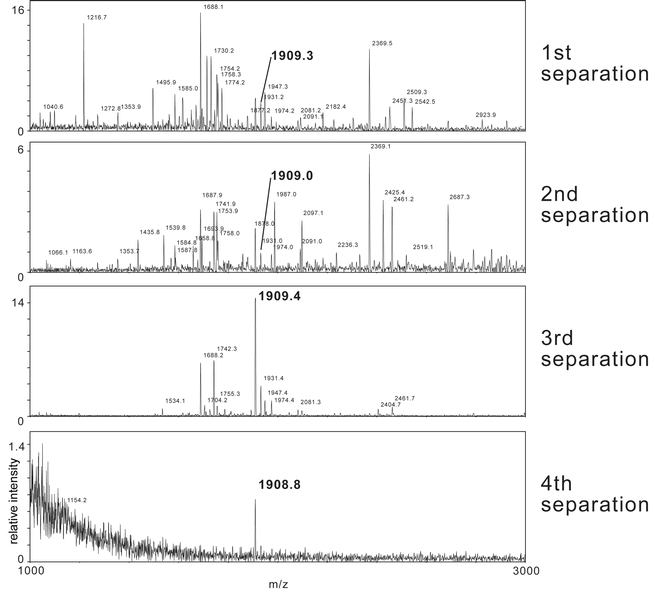
MALDI-TOF mass spectra of Leucophaea maderae pigment-dispersing factor immunoreactive fractions during the separation process using four different column/mobile phase combinations. Peaks corresponding to Lem-PDF (theoretical mass: [M+H]+ 1909.08 Da) are marked with an arrow. Masses given as [M+H]+.
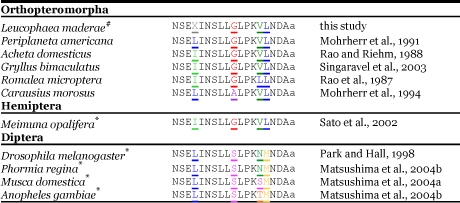
Fractions 36-38 were pooled, and the PDF-like immunoreactive material was isolated in three further chromatographic steps. In each step, HPLC peaks were collected manually and assayed in ELISA and MALDI-TOF MS (Fig. 4). Only the respective immunoreactive fractions were further purified. After the 4th column, the immunoreactive peak was found to contain only one substance with the protonated mass of 1908.8 Da in MALDI-TOF MS and a retention time of 42-43 min. After the fourth separation, Drm-PDF, Acd-PDF and Pea-PDF were separated under identical conditions on the 4th column and were found to have retention times of 38-39, 43, and 44 min respectively. The total amount of PDF-like immunoreactivity isolated from a thousand brains was estimated to be 60 pmol (based on ELISA) and 200 pmol (based on peak area in HPLC) respectively, which would equal 60 or 200 fmol/brain. About 12 pmol (based on ELISA) of the isolated material was used for Edman-sequencing, which failed although enough material should have been present. The reason for this could be that either the amount of PDF-like immunoreactive material was overestimated by ELISA and HPLC, or that the material could not be recovered from the final tube. However, it was possible to fragment the PDF-like immunoreactive material by ESI-QTOF MS (Fig. 5). Based on the fragment series, Lem-PDF has the sequence NSEXXNSXXGXPKVXNDAa, where X is either L or I which have the same mass and can thus not be distinguished by ESI-QTOF MS. PDH/PDFs share a very conservative 18 amino acid sequence (see Rao, 2001). Sequence comparison of insect pigment-dispersing factors shows that only the amino acid positions 4, 10, 14, and 15 are variable (see Table 1), corresponding to X, G, V, and X in L. maderae. Position 15 is either L (in Orthopteromorpha and Hemiptera) or M (in Diptera). Given the high sequence conservation of pigment-dispersing factor and the grouping of L. maderae within the Orthopteromorpha, it seems reasonable to suggest that Lem-PDF has the sequence NSEXINSLLGLPKVLNDAa and thus is identical to either Pea-PDF or Acd-PDF. The similar retention time of Lem-PDF with Acd-PDF but not Pea-PDF after the fourth column suggests that Lem-PDF is identical to Acd-PDF.
Figure 5.
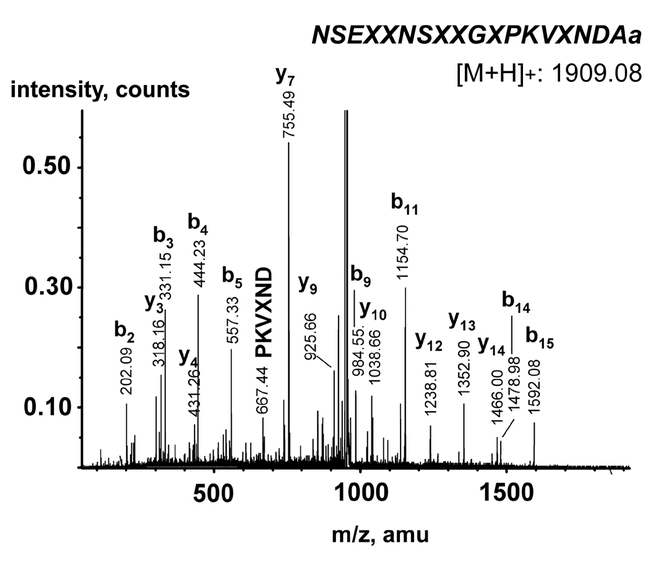
Mass spectrum of the isolated Lem-PDF fragmented by ESI-Q-TOF. The near-to-complete b+1 and y+1 ion fragment series are visible. X = Leu or Ile.
Table 1.
Insect PDF sequences.
Quantification of PDF-like immunoreactivity by immunofluorescence staining
β-PDH immunostaining intensities were compared in different part of the brain at different time points under a 12/12 light/dark cycle. Based on a previous morphological description (Reischig and Stengl, 2002; Fig. 6), five regions were separately analyzed where the pigment-dispersing factor neurons terminate, and three regions where their cell bodies are located. In general, staining indices varied in the cell bodies, but not in the terminals. The posterior and the anterior medulla cell bodies had a tendency for higher staining indices than that of the terminals (Fig. 6).
Figure 6.
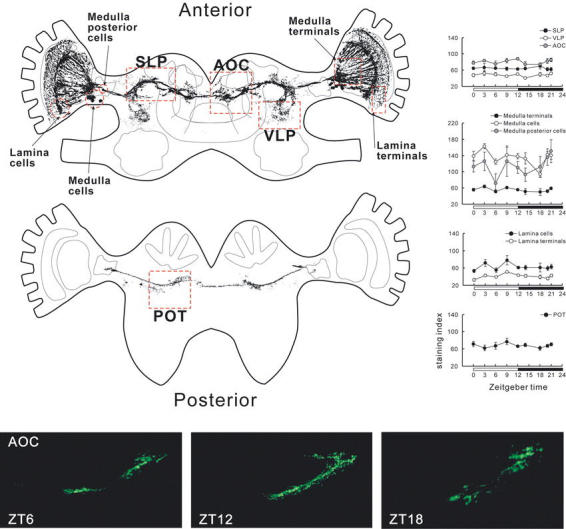
Spatial analysis of PDF-immunoreactivity at different ZT times under LD conditions. Staining intensities were measured in nine areas of the brain (red squares in the top-left drawing of the PDF-immunoreactive neurons). The graphs show the average standardized staining indices ± s.e.m. The bottom panel shows representative immunostainings in the anterior optic commissure region at different ZT times. Note that the morphology of the neurons is slightly different due to different angles of the individual sections, but staining intensity was almost the same at the different time points. AOC, anterior optic commisure; POT, posterior optic tract; SLP, superior lateral protocerebrum; VLP, ventro-lateral protocerebrum. To draw the PDF-immunoreactive neurons, brain sections were photographed and converted into semi-tranparent pictures in Corel Photopaint. The converted images were superimposed, and background was deleted with a threshold command in Corel Photopaint. The schematic brain structures were drawn in Corel Draw, referring to Reischig and Stengl (2002).
In all protocerebral regions with branches of PDF-immunoreactive neurons (superior lateral protocerebrum, ventro-lateral protocerebrum, posterior optic tract, and the anterior optic commissure), the average staining indices did not change significantly during day and night, (one-way ANOVA, P > 0.05; n = 4 - 25 for each region, Fig. 6). The staining index did not fluctuate significantly in the anterior medulla cell bodies (ANOVA, P > 0.05). However, the staining index was remarkably low at ZT 18 (middle of the dark phase) in the anterior medulla cell bodies (N = 5). The staining index of the posterior medulla cell bodies decreased remarkably at ZT 6 (middle of the light phase; N = 6; Fig. 6), but significant fluctuations were not detected by one-way ANOVA (P > 0.05). The cluster of cell bodies and the ventral half of the terminals of the lamina pigment-dispersing factor neurons were also tested. Although the variation of the staining index at each time point was small, there was no significant fluctuation in the lamina terminals or cell bodies (ANOVA, P > 0.05).
Discussion
Due to its robust circadian locomotory activity (Roberts 1960), L. maderae has been a preferred insect in circadian research. Results of detailed studies suggest that the neuropeptide pigment-dispersing factor plays a prominent role in the circadian pacemaker circuits in the brain of this cockroach. In this study, an authentic orthopteromorphan pigment-dispersing factor is described that is present in the brain of L. maderae. Based on mass spectrometric data and sequence homology found within the Orthopteromorpha, Lem-PDF should have the sequence NSEXINSLLGLPKVLNDAa, where X is either leucine or isoleucine. Lem-PDF thus appears to be identical to the pigment-dispersing factor of either the cockroach P. americana or the crickets Acheta domesticus and Gryllus bimaculatus (see Table 1). Unfortunately, we did not obtain enough material for Edman sequencing that would have revealed whether leucine or isoleucine is in position 4. Since I and L are similar in physico-chemical properties and size, we predict that a substitution of position 4 of Lem-PDF with either I or L will only slightly change the peptide's bioactivity. Molecular cloning based on sequence homology of the mRNA for β-PDH and pigment-dispersing factor has recently led to the identification of pigment-dispersing factor in several insect species (Chuman et al., 2002; Sato et al., 2002; Matsushima et al., 2004a, b), and could also be used to clarify the full sequence of Lem-PDF. On the other hand, the occurrence of the pigment-dispersing factor peptides predicted from cDNA cloning still has to be verified biochemically in those insects where only the genes were cloned.
In the diurnal D. melanogaster, pigment-dispersing factor is involved in the regulation of locomotory activity and plays a role as an output factor of the circadian pacemaker in the brain, (Renn et al., 1999; Helfrich-Förster et al., 2000) where it appears to be released during the light phase (Park et al., 2000). L. maderae shows locomotory activity mainly during the dark phase.
Since pigment-dispersing factor seems to play an important role in controlling locomotor activity in L. maderae (Stengl and Homberg, 1994, Reischig and Stengl, 2003), we hypothesized that Lem-PDF might be released during the dark phase. A release of pigment-dispersing factor during the night was also suggested for G. bimaculatus, where pigment-dispersing factor appears to enhance the photo-responsiveness of the medulla bilateral neurons (Saifullah and Tomioka, 2003). Given a constant transcription and translation rate for pigment-dispersing factor, a release would be predicted during the dark phase leading to a circadian change of the amount of Lem-PDF at least in some parts of the PDF- immunoreactive neurons of the brain. To detect such changes quantification of standardized immunofluorescence was used since this method was successfully employed in studies on D. melanogaster (Park et al., 2000) and allows for good spatial resolution.
Although immunoassays may provide a more sensitive and quantitative method, they can only be used to quantify averaged amounts from different neuronal compartments, whereas changes in the amount of pigment-dispersing factor might occur only at, for example, output sites as was shown for D. melanogaster (Park et al., 2000).
By quantifying the intensity of immunofluorescence in brain sections, we could however not find significant daily changes of the amount of PDF-immunoreactivity in different regions of the brain. This might indicate that Lem-PDF is not released in a circadian fashion. Yet, it is also possible that the amount of released pigment-dispersing factor is simply too small to bring about a change of PDF-immunoreactivity that can be detected by immunostaining. Alternatively, it is possible that PDF-cells in the lamina and medulla release or produce pigment-dispersing factor under circadian control but with a different phase than other pigment-dispersing factor cells even within the same cluster. It is noteworthy that the PDF-immunoreactive neurons of the anterior medulla seem to take part in different clock circuits (Reischig et al., 2004). In D. melanogaster, the two subpopulations of the PDF-expressing LNvs, which serve in different circuits, show cell-specific differences in the phase angles of the nuclear accumulation of the clock proteins PERIOD and TIMELESS (Shafer et al., 2002). Since we were not able to individually identify single cells within the clusters due to variable cell positions between preparations, we averaged the measurements for each cluster. This averaging might have masked detection of circadian changes of PDF-immunoreactivity in individual cells. Combined, these results are not in favor of a strong-phase coupling of daily pigment-dispersing factor release or production between the pigment-dispersing factor cells within the different clusters, and implicate that there is no drastic daily depletion of PDF-immunoreactivity in the cell bodies or terminals of the pigment-dispersing factor cells.
In addition to a possible role in controlling locomotor activity, Lem-PDF seems to have the important function to couple the bilaterally paired pacemaker centers of the accessory medullae (Petri and Stengl, 1997; Reischig and Stengl, 2002; Reischig et al., 2004). When injected into the vicinity of the accessory medullae, Arg13-Acd-PDF induced a maximal and significant phase delay at circadian time 8:50, i.e. during the late subjective day. Interestingly, one of the two distinct changes in PDF-like immunoreactivity described here occurred at ZT 6 in the PM cell bodies, i.e. at a time point about 2-3 h in advance of the circadian time when pigment-dispersing factor has maximal phase-shifting effects. It is, however, not clear from our data whether the two observed distinct changes in PDF- immunoreactivity are of biological significance. Further investigations may reveal whether they represent a distinct endogenous circadian rhythm or are caused exogenously by the LD-cycle.
In conclusion, these results show that L. maderae expresses an orthopteromorphan-type pigment-dispersing factor, the amount of which is apparently not as drastically depleted or reduced due to peptide release at the terminals as reported for D. melanogaster.
Acknowledgments
We would like to thank Dick R. NÄssel (Stockholm) for providing facilities and for critical discussion of the manuscript, Friedrich Buck (UKE Hamburg) for Edman sequencing, Anne Starck (Stockholm) for excellent help during dissections, Heinrich Dircksen (Stockholm) for the kind gift of antiserum and Paul Taghert (St. Louis) for the kind gift of flies. We wish also to thank K. Ranga Rao (Pensacola) and the late John Riehm (Pensacola) for providing the synthetic PEA- and Acheta-PDFs that were used in this study, and the unknown referees for valuable comments. Supported by the Karl Trygger's Foundation, to D. R. Nässel.
References
- Chuman Y, Matsushima A, Sato S, Tomioka K, Tominaga Y, Meinertzhagen IA, Shimohigashi Y, Shimohigashi M. cDNA cloning and nuclear localization of the circadian neuropeptide designated as pigment-dispersing factor PDF in the cricket Gryllus bimaculatus. Journal of Biochemistry. 2002;131:895–903. doi: 10.1093/oxfordjournals.jbchem.a003180. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Muller A, Keller R. Crustacean cardioactive peptide in the nervous-system of the locust, Locusta-migratoria - an immunocytochemical study on the ventral nerve cord and peripheral innervation. Cell and Tissue Research. 1991;263:439–457. [Google Scholar]
- Fingerman M, Rao KR, Bartell CK. A proposed uniform method of reporting response values for crustacean chromatophorotrophins: the standard integrated response. Experientia. 1967;23:962. doi: 10.1007/BF02136250. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Täuber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A. Ectopic expression of the neuropeptide Pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. Journal of Neuroscience. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microscopy Research and Technique. 2003;62:94–102. doi: 10.1002/jemt.10357. [DOI] [PubMed] [Google Scholar]
- Homberg U, Würden S, Dircksen H, Rao KR. Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell and Tissue Research. 1991;266:343–357. [Google Scholar]
- Homberg U, Reischig T, Stengl M. Neural organization of the circadian system of the cockroach Leucophaea maderae. Chronobiology International. 2003;20:577–591. doi: 10.1081/cbi-120022412. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Sato Y, Takeda Y, Yokotani S, Nose T, Tominaga Y, Shimohigashi M, Shimohigashi Y. cDNA cloning of the housefly pigment-dispersing factor (PDF) precursor protein and its peptide comparison among the insect circadian neuropeptides. Journal of Peptide Science. 2004a;10:82–91. doi: 10.1002/psc.511. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Yokotani S, Lu X, Sumida K, Honda T, Sato S, Kaneki A, Takeda Y, Chuman Y, Ozaki M, Asai D, Nose T, Onoue H, Ito Y, Tominaga Y, Shimohigashi Y, Shimohigashi M. Molecular cloning and circadian expression profile of insect neuropeptide PDF in black blowfly, Phormia regina. Letters in Peptide Science. 2004b;10:419–430. [Google Scholar]
- Mohrherr C, Rao KR, Riehm JP. Characterization of a pigment-dispersing factor from the American cockroach. Society for Neuroscience abstracts. 1991;17:114.6. [Google Scholar]
- Mohrherr C, Maruska K, Raabe M, Riehm JP, Rao KR. Primary structure of a pigment-dispersing factor from the stick insect, Carausius morosus. Society for Neuroscience abstracts. 1994;20:914. [Google Scholar]
- Nässel D, Shiga S, Mohrherr CJ, Rao KR. Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. Journal of Comparative Neurology. 1993;331:183–198. doi: 10.1002/cne.903310204. [DOI] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J, Pittendrigh CS. Central nervous system control of circadian rhythmicity in the cockroach. III. The optic lobes, locus of the driving oscillation? Zeitschrift für Vergleichende Physiologie. 1968;58:14–46. [Google Scholar]
- Park J, Hall JC. Isolation and chronobiological analysis of a neuropeptide pigment-dispersing factor gene in Drosophila melanogaster. Journal of Biological Rhythms. 1998;13:219–228. doi: 10.1177/074873098129000066. [DOI] [PubMed] [Google Scholar]
- Park J, Helfrich-Förster C, Lee G, Liu L, Rosbash M, and Hall JC. 2000 Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proceedings of the National Academy of Science. USA. 97:3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M, Eklund MB, Dircksen H, Muren JE, Nässel DR. Pigment-dispersing factor in the locust abdominal ganglia may have roles as circulating neurohormone and central neuromodulator. Journal of Neurobiology. 2001;48:19–41. doi: 10.1002/neu.1040. [DOI] [PubMed] [Google Scholar]
- Petri B, Stengl M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. Journal of Neuroscience. 1997;17:4087–4093. doi: 10.1523/JNEUROSCI.17-11-04087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnson L, Tarr GE. Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. Journal of Biological Chemistry. 1987;262:2672–2675. [PubMed] [Google Scholar]
- Rao KR, Riehm JP. Pigment-dispersing hormones: a novel family of neuropeptides from arthropods. Peptides. 1988;9(Suppl. 1):153–159. doi: 10.1016/0196-9781(88)90239-2. [DOI] [PubMed] [Google Scholar]
- Rao KR. Crustacean pigmentary-effector hormones: chemistry and functions of RPCH, PDH, and related peptides. American Zoologist. 2001;41:364–379. [Google Scholar]
- Reischig T, Stengl M. Optic lobe commissures in a three-dimensional brain model of the cockroach Leucophaea maderae: a search for the circadian coupling pathways. Journal of Comparative Neurology. 2002;443:388–400. doi: 10.1002/cne.10133. [DOI] [PubMed] [Google Scholar]
- Reischig T, Stengl M. Ectopic transplantation of the accessory medulla restores circadian rhythmic behaviour in arrhythmic cockroaches (Leucophaea maderae) Journal of Experimental Biology. 2003;206:1877–1886. doi: 10.1242/jeb.00373. [DOI] [PubMed] [Google Scholar]
- Reischig T. 2003 Lesions of PDH-immunoreactive medulla neurons abolishes circadian rhythmic locomotor activity in the cockroach Leucophaea maderae. Dissertation, Philipps-University. Marburg. 65–78. [Google Scholar]
- Reischig T, Petri B, Stengl M. Pigment-dispersing hormone (PDH)-immunoreactive neurons form a direct coupling pathway between the bilaterally symmetric circadian pacemakers of the cockroach Leucophaea maderae. Cell and Tissue Research. 2004;318:553–564. doi: 10.1007/s00441-004-0927-1. [DOI] [PubMed] [Google Scholar]
- Renn S, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Saifullah A, Tomioka K. Pigment-dispersing factor sets the night state of the medulla bilateral neurons in the optic lobe of the cricket, Gryllus bimaculatus. Journal of Insect Physiology. 2003;49:231–239. doi: 10.1016/s0022-1910(02)00270-6. [DOI] [PubMed] [Google Scholar]
- Sato S, Chuman Y, Matsushima A, Tominaga Y, Shimohigashi Y, Shimohigashi M. A circadian neuropeptide, pigment-dispersing factor-PDF, in the last-summer cicada Meimuna opalifera: cDNA cloning and immunocytochemistry. Zoological Science. 2002;19:821–828. doi: 10.2108/zsj.19.821. [DOI] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. Journal of Neuroscience. 2002;22:5946–5954. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravel M, Fujisawa Y, Hisada M, Saifullah ASM, Tomioka K. Phase shifts of the circadian locomotor rhythm induced by pigment-dispersing factor in the cricket Gryllus bimaculatus. Zoological Science. 2003;20:1347–1354. doi: 10.2108/zsj.20.1347. [DOI] [PubMed] [Google Scholar]
- Stengl M, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. Journal of Comparative Physiology A. 1994;175:203–213. doi: 10.1007/BF00215116. [DOI] [PubMed] [Google Scholar]


