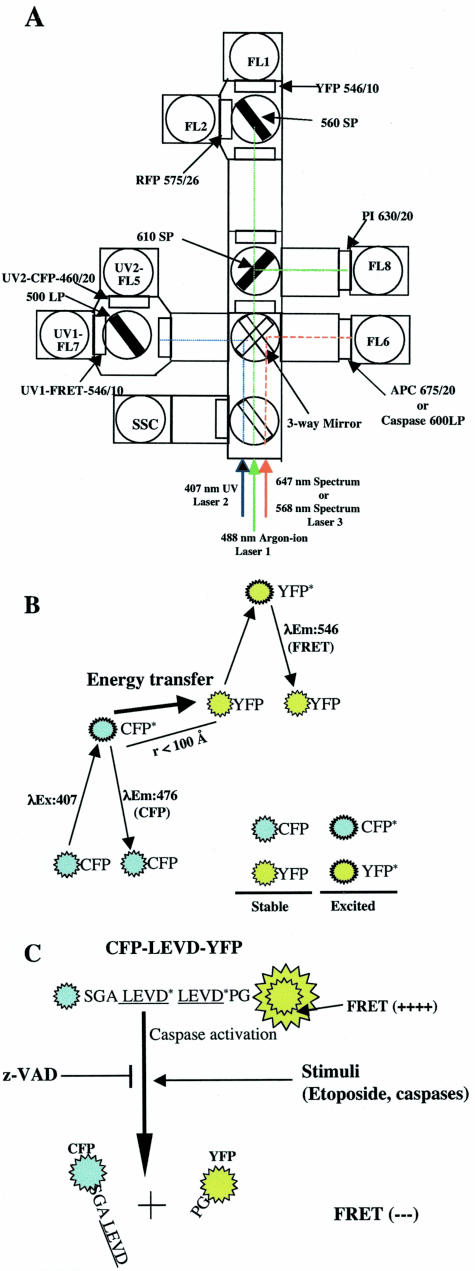
Figure 1.
FRET and its use for measurement of caspase activity by flow cytometry. A: Optical configuration of the BD FACSDiVa triple-laser system (488-nm, 407-nm, and 647-nm laser lines) and DakoCymation MoFlo used to measure spatially separated FRET, CFP, YFP, RFP, PI, and APC emission signals as well as red fluorescence that are emitted from a caspase-sensitive substrate or FLICAs. A 488-nm line emitted from the argon-ion laser was used to excite YFP (FL1), RFP (FL2), and PI (FL8). A 546/10 band-pass filter was used with a 560 short-pass dichroic filter to detect the acceptor YFP signal (FL1). A 575/26 band-pass filter and a 630/20 band-pass filter were used to detect RFP (FL2) and PI (FL8) signals, respectively. RFP, PI, and YFP signals were detected in the first laser pathway. A 460/20 and a 546/10 band-pass filter in the second laser pathway was used with a 500 long-pass dichroic filter to detect the UV-excited CFP signal and the FRET or “sensitized” YFP signal, respectively (FL5 and FL7). A 675/20 band-pass filter was used to detect the 647-nm line-excited APC signals in spectrum laser pathway (laser 3). When caspase activities in living cells were determined by using the fluorogenic substrate PhiPhiLux-G2D2 or the FLICAs, SR-VAD-FMK, or SR-DEVD-FMK, the substrate or FLICA was excited by 568-nm line emitted from the tunable Spectrum laser (the third laser) and its red emission was collected by a 600 long-pass filter (FL6). B: FRET between CFP and YFP when they are physically in close proximity. CFP molecules are excited by the 407-nm line emitted from the krypton laser. These excited CFP molecules revert to the resting state by either directly emitting energy or transferring energy to closely approximated acceptor YFP molecules. The latter process leads to direct excitation of YFP molecules and emission of YFP photons as FRET signals. C: Diagram showing that FRET can be used to monitor cellular caspase activity. A 14-amino acid spacer that has two caspase cleavage sites (LEVD) was inserted between CFP and YFP. In cells with caspase activity, there is a loss of FRET signals because of the cleavage of the probe and the physical separation of the CFP and YFP moieties. Several agents that induce or inhibit caspase activity were examined using this caspase-sensitive probe and flow cytometry.