Abstract
Tranilast, an antiallergic medication, is a very promising inhibitor of restenosis after balloon angioplasty. Tranilast can prevent the proliferation and migration of smooth muscle cells by activating the gene expression of p21, a strong cyclin/cyclin-dependent kinase (CDK) inhibitor, and by arresting cell growth at the G0/G1 phase.
The signaling pathway of Tranilast in regulating p21 is to our best interest and is elucidated in the present study. The major emphasis was weighted on exploring the regulatory effects of Tranilast on promoter activity of p21.
By serial deletion analysis, the sequence between −74 and −83 bp of the p21 promoter, previously identified as the transforming growth factor-β (TGF-β)-response element, was found sufficient, where as most of the promoter region 5′ to −111 bp was found unnecessary for the transcriptional activation of p21 by both TGF-β1 and Tranilast.
Tranilast was also found to induce phosphorylation of Smad2 (a cytoplasmic signaling molecule essential for mediating TGF-β signal transduction). Transfection of ΔkTβRII, a truncated form of TGF-β type II receptor known to exert a dominant-negative effect on TGF-β signaling, was found to suppress the signaling of both Tranilast and TGF-β1 to a similar extent.
These results suggested that induction of p21 by Tranilast might be closely related to TGF-β signal transduction pathway.
Keywords: Restenosis, p21 induction, TGF-β signaling, TGF-β response element
Introduction
Restenosis remains a serious long-term complication after percutaneous transluminal coronary angioplasty (PTCA), occurring in approximately 30–40% of patients (Nobuyoshi et al., 1988; Fischman et al., 1994; Serruys et al., 1994; Savage et al., 1998). Proliferation and migration of vascular smooth muscle cells (SMCs) play a major role in the development of restenosis and in the progression of atherosclerosis (Ross, 1993). Arterial injury induces the migration of SMCs into the intimal layer of the vessel wall, where they proliferate and synthesize extracellular matrix components. Many growth factors induce the proliferation of vascular SMCs in vitro and in vivo. Among them, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) are important regulators of vascular SMCs through their well-defined actions as strong mitogens and potent chemoattractants. Therefore, it is anticipated that factors or agents that block cell signals affecting the proliferation and migration of vascular SMCs may be effective in preventing restenosis.
Pharmacological approaches to prevent restenosis have been largely disappointing (Franklin & Faxon, 1993). However, recent clinical studies have demonstrated that oral Tranilast decreases the rate of angiographic restenosis. Tranilast (N-3,4-dimethoxycinnamoyl anthranilic acid) is an antiallergic drug that has been widely used in Japan since the 1980s to treat allergic rhinitis and bronchial asthma and to prevent keloid formation after skin injury. Because the process of keloid formation after skin injury and that of restenosis after PTCA have common features, Tranilast was tested in several large clinical trials for the prevention of restenosis. The TREAT-2 study (The Second Tranilast REstenosis following Angioplasty Trial), a double-blind, randomized, multicenter trial (a total of 297 patients) found that Tranilast (600 mg day−1 for 3 months) reduced the rate of angiographic restenosis at 3 months post-PTCA from 41.9 to 25.9%, a reduction of 38.2% (P=0.012) (Tamai et al., 2002). In patients undergoing directional coronary atherectomy (Kosuga et al., 1997), the relative reduction in the 1-year event rate of restenosis was 60% in patients treated with Tranilast versus control (12.5 versus 31.6%, P<0.03).
Tranilast has been found to inhibit both in vitro and in vivo the proliferation and migration of human and rat aortic SMCs (Miyazawa et al., 1995; Fukuyama et al., 1996). These activities may be very important in the prevention of restenosis (Miyazawa et al., 1995; Fukuyama et al., 1996). Tranilast was found to arrest human coronary SMC proliferation at the G0/G1 phase of the cell cycle (Kusama et al., 1999). Recent studies have demonstrated that Tranilast increased p21waf1 CDK inhibitor and p53 tumor suppressor factor (Kusama et al., 1999; Takahashi et al., 1999). The binding of p21 to CDK2/CDK4 inhibits CDK2/CDK4 kinase activity thereby stopping cell cycle at the G0/G1 phase. Other CDK inhibitors such as p16INK (p16) and p27kipl (p27) were not induced after Tranilast treatment (Takahashi et al., 1999). These results suggested that Tranilast inhibited the proliferation of SMCs mainly through induction of p21 and p53.
Although Tranilast can induce the expression of p21 in SMCs, the molecular mechanism underlying this induction is not clearly defined. As promoter activation is closely associated to gene expression, we therefore investigated the effects of Tranilast on p21 promoter activity at the molecular level in the present study. The effect of this drug on the p21 signaling pathway can then be further elucidated.
Methods
Cell culture
HaCaT cell is a human keratinocyte cell line that is growth-inhibited by TGF-β, and whose p21 is upregulated by TGF-β (Datto et al., 1995b). This cell line is a gift kindly provided by Dr Fusenig (Boukamp et al., 1988). HaCaT cells were maintained in Dulbecco's modified Eagle's medium/Ham's nutrient mixture F-12 (1 : 1) (DMEM-F12) (Life Technologies, Inc., Rockville, MD, U.S.A.) and 10% fetal bovine serum (FBS) (Biological Industries, Beit Haemek, Israel) at 37°C and in an atmosphere of 5% CO2 in air (standard conditions of incubation).
Plasmids
Plasmids p21P, p21P 93-S, and p21P 93-S mut#1–6 as well as serial deletion constructs of p21P were gifts of Dr Xiao-Fan Wang (Datto et al., 1995b). The 2.4-kb full-length p21 promoter sequence containing the p21 cDNA start site at its 3′-end was subcloned into the HindIII site of the luciferase reporter vector, pGL2-basic (Promega, Madison, WI, U.S.A.), to create p21P. Plasmid p21P-Δ1.9 carries a 500-bp fragment of p21P resulting from deletion of 1900 bp from the 5′-end. Plasmid p21PSma carries a fragment consisting of the last 111 bp of the 3′-end of the promoter. Plasmid p21PSmaΔ1 carries a fragment consisting of the last 61 bp of the 3′-end of the promoter. Plasmid p21PSmaΔ2 carries a fragment resulting from an internal 50 bp deletion (from −61 to −111 bp) of the promoter. Plasmid p21P 93-S carries the p21 promoter sequence between −62 and −93 bp. Plasmid p21P 93-S mut#1–6 each carries the p21 promoter sequence between −34 and −93 bp altered by serial site-directed mutagenesis to create a mutated sequence of 10 consecutive bases. The oligonucleotides used in the mutagensis reactions have been reported (Datto et al., 1995b) and described as follows: p21P 93-S mut#1, CCTCAAGGAGGCGGGGTTTCTAGATATCGAATTCCTG. p21P 93-S mut#2, CCCGGGCCCGCCTCAGTTCTAGATAACCCGCGCTC. p21P 93-S mut#3, CCGCCCGCCCGGATTTCTAGAGAGGAGGCGGG. p21P 93-S mut#4, CCTGATATACAACCGTATTCTAGAAGCCCGCCTCAAGGCGGG. p21P 93-S mut#5, CAGCGCGGCCCTGACGCGTCTAGACCCCGCCCGG. p21P 93-S mut#6, GCTGGCGCAGCTCAGCATCTAGACAATATACAACCGCC. ΔkTβRII is a truncated form of TGF-β type II receptor cloned in pSV-Sport 1 vector (GIBCO BRL, Grand Island, NY, U.S.A.) (Brand et al., 1993).
Determination of cell number
HaCaT cells were seeded into 96-well plates at a concentration of 1 × 104 cells per well and cultured overnight in the medium described above. The medium was then replaced with DMEM-F12 with 10% FBS containing Tranilast (0, 30, 100, 300, or 1000 μM), and incubated under standard incubation conditions for 3 days. The cells were washed twice with phosphate-buffered saline (PBS), 100 μl of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) (Sigma, St Louis, MO, U.S.A.) was added to each well, and the plates were incubated at 37°C for 4 h. Finally, MTT was replaced by 100 μl of dimethyl sulfoxide (DMSO) and incubated for a further 5 min. The absorbance of the samples was measured at 570 nm using a microtiter plate reader.
Cytotoxic assay
HaCaT cells were treated with Tranilast in the same way as describe above. Following 72 h of incubation, lactate dehydorgenase (LDH) release into the medium was measured at 570 nm using a microtiter plate reader according to the manufacture's protocol (LDH-cytotoxic test; Wako, Osaka, Japan).
Transfection and luciferase assay
HaCaT cells were plated at a density of 1 × 105 cells per well in 12-well plates in DMEM-F12 and 10% FBS overnight so that they were 70–90% confluent on the day of transfection. Cells were transfected with 0.5 μg of each p21P reporter constructs, or cotransfected with 0.5 μg of p21P and 0.5 μg of ΔkTβRII plasmid and incubated with 2.5 μl of lipofectamine in 300 μl of Opti-MEM according to the manufacturer's instructions (Life Technologies, Inc.) under standard conditions for 3 h. After 3 h of incubation, the medium was replaced with DMEM-F12 containing 10% FBS followed by overnight incubation under standard conditions. The cells were treated with growth medium in the presence or absence of 100 μM Tranilast (gift from Lotus Pharmaceutical Co., Taipei, Taiwan) or 100 pM TGF-β1 for 20 h. The cells were harvested and the luciferase activity was determined using a Berthold luminometer as described previously (Charng et al., 1996).
Western blotting
HaCaT cells (1.5 × 106 cells per 100 mm tissue culture dish) were plated in DMEM-F12 containing 10% FBS and incubated overnight under standard conditions. The cells were treated with 100 pM TGF-β1 or 100 μM Tranilast and then harvested every 15 min for 45 min. The harvested cells were solubilized in 500 μl of lysis buffer (150 mM NaCl, 20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 0.5% Triton X-100, 1 mM dithiothreitol, Pefabloc (1 mg ml−1), leupeptin (10 μg ml−1), pepstatin (10 μg ml−1), aprotinin (1 μg ml−1), NaF (50 mM), sodium orthovanadate (1 mM), and okadaic acid (1 μM)) and 20 μg of total protein from each cell lysate was fractionated on a 12% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane, and then probed with primary antibody against phospho-Smad2 (1 : 500 dilution of a rabbit polyclonal IgG anti-phospho-Smad2 (Ser 465–467) antibody Upstate Biotechnology, Lake Placid, NY, U.S.A.). Bound primary antibody was detected with horseradish peroxidase (HRP)-labeled anti-rabbit secondary antibody (1 : 3000 dilution; ICN Pharmaceuticals, Inc., Costa Mesa, CA, U.S.A.), and developed using the enhanced chemiluminescence system (NEN™ Life Science Products, Inc., Boston, MA, U.S.A.).
ELISA analysis of TGF-β1
ELISA kits for TGF-1 were purchased from Amersham Pharmacia Biotech (Buckinghamshire, U.K.). The assay procedures were based on the protocols provided by the manufacturer. Briefly, 3 × 104 of HaCaT cells seeded in 12-well plates overnight followed by 30 min incubation of 30, 60, 100 and 300 μM of Tranilast with DMSO as the control. About 0.5 ml of cell culture supernatant was added with 0.1 ml 1 N HCl and incubated at room temperature for 10 min followed by addition of 0.1 ml 1.2 N NaOH and 0.5 M HEPES. Each reaction mixture was placed into ELISA plates. Following 1 h of incubation at 37°C, the conjugated complexes were washed 4 times with wash buffer containing 0.01 M phosphate buffer saline pH7.4 and 0.2% Tween 20. Antibodies were then added to the sample complexes for 1 h incubation at 37°C followed by reaction with biotinylated second antibodies for 1 h at 37°C. Additional conjugation with streptavdin–HRP was carried out for 30 min at 37°C. After washing 4 times with wash buffer, 3,3′,5,5′-tetramethylbenzidine/hydrogen peroxide solution in 20% (v v−1) dimethylformamide as substrates was added to plates for 30 min of incubation at room temperature. The optical density reading for each sample was then performed at 450 nm by a spectrophotometer (Beckman, U.S.A.). The actual concentrations were then converted from the corresponding standards.
Statistics
Data are expressed as mean±s.e. Statistical analysis was conducted using unpaired-Student's t-test. A P-value <0.05 was considered statistically significant.
Results
Tranilast inhibits the proliferation of HaCaT cells in a dose-dependent manner
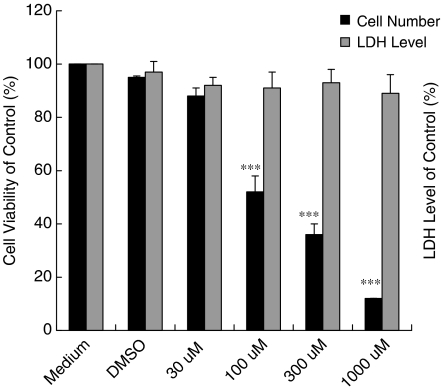
To determine whether Tranilast possesses inhibitory effects on cell proliferation, the MTT assay was performed in the present study. Figure 1 shows that Tranilast (30, 100, 300, or 1000 μM) inhibited the proliferation of HaCaT cells in a dose-dependent manner. This inhibition was not a result of direct cell toxicity caused by Tranilast, because treatment with Tranilast did not increase the level of LDH in the culture medium (Figure 1).
Figure 1.
Effect of Tranilast on proliferation of HaCaT cells. HaCaT cells (1 × 104 per well) in 96-well plates cultured overnight were treated with growth media containing Tranilast (0, 30, 100, 300, or 1000 μmol l−1 (μM)). Cell number was determined by MTT assay. Tranilast is seen to inhibit the proliferation of HaCaT cells in a dose-dependent manner. Cytotoxicity manifested as LDH level was also determined under the effects of Tranilast. Each value represents the mean±s.d. (n=6). ***P<0.001 as compared to DMSO control in each group.
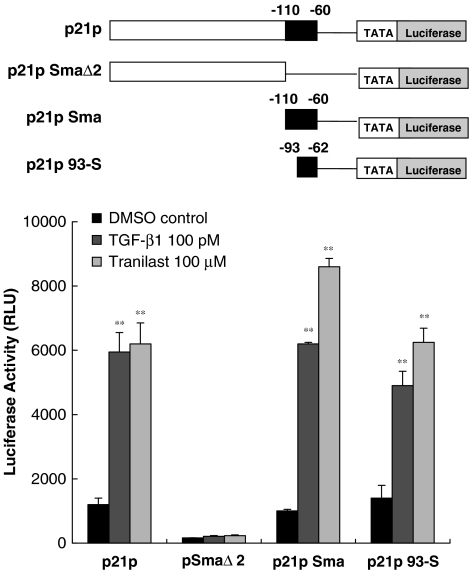
Tranilast response element is located between −60 and −110 bp of the p21 promoter
To define the region of the p21 promoter required for transcriptional activation by Tranilast, each promoter construt with serial deletion was transfected into HaCaT cells, which were previously shown to be growth-inhibited by 100 or 300 μM Tranilast. The transcriptional activation of these promoter constructs was manifested by the luciferase activity. Cells transfected with the full-length p21 promoter reporter, p21P, or the deletion construct p21PSma were activated about four-fold by Tranilast, indicating that the Tranilast response element is located in the small 3′ region of the p21 promoter. In cells transfected with p21PSmaΔ2 (i.e., p21P with a 50-bp deletion from −60 to −110 bp), the luciferase response to Tranilast was completely abolished (Figure 2). However, the cells transfected with the p21p93-S construct, that is, the promoter region between −62 and −93 bp, was significantly activated by Tranilast, indicating that this region was sufficient for activation (Figure 2). This finding is in consistent with our preliminary data demonstrating that the Tranilast response region is in the 3′-end (–62 to −93 bp) of p21 promoter.
Figure 2.
Deletion analysis of the p21 promoter. Full-length and different deletion constructs of p21 promoter reporter were transfected into HaCaT cells and the cells subsequently treated with Tranilast and TGF-β1. Cell lysates were measured for luciferase activity in relative light units. The results indicated that the sequence between −60 and −110 bp of p21 promoter is necessary for activation by both TGF-β1 and Tranilast. Each value represents the mean±s.d. (n=6). **P<0.01 as compared with DMSO control in each group.
To demonstrate that the TGF-β response element was located in a similar region to the Tranilast response region, we measured luciferase activity in the above transfected cells with 100 pM TGF-β stimulation. As seen in Figure 2, TGF-β and Tranilast stimulated parallel responses, suggesting that both response elements were either located at the same region or they overlapped and spanned the region between −62 and −93 bp of the promoter.
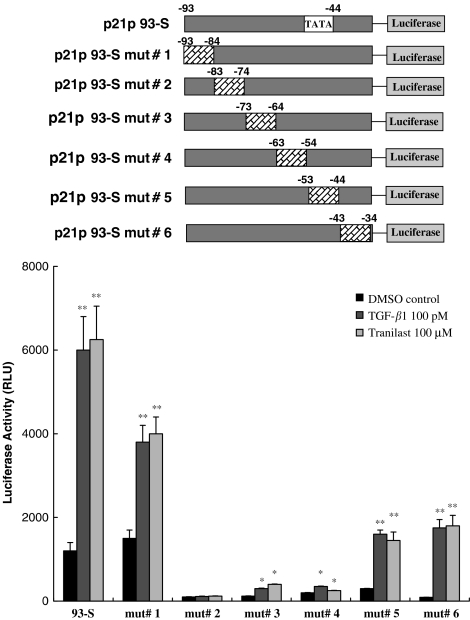
Mutational analysis of p21 promoter
To define more precisely where on the 30-bp region the Tranilast and TGF-β response elements were located, a serial site-directed mutagenesis of this region was performed and tested for the induction of luciferase activity by Tranilast or TGF-β. As shown in Figure 3, the luciferase activity of HaCaT cells transfected with constructs of mutants #1–#6 of the p21P93-S sequence demonstrated differential responses to TGF-β or Tranilast. This finding suggested that the region between −74 and −83 bp was most critical for Tranilast and TGF-β responses. This same region was previously identified as the TGF-β response element (TβRE) (Datto et al., 1995b).
Figure 3.
Mutational analysis of the p21 promoter. Mutants #1–#6 were generated from different site-directed mutagenesis of the p21p93-S sequence. HaCaT cells were transfected with these mutants and then treated with TGF-β1 or Tranilast. The responses to TGF-β1 and Tranilast were almost identical. The sequences between −74 and −83 bp, a previously defined TGF-β-response element (TβRE), were essential to both TGF-β1- and Tranilast-induced activation. Each value represents the mean±s.d. (n=6). *P<0.05; **P<0.01 as compared to DMSO control in each group.
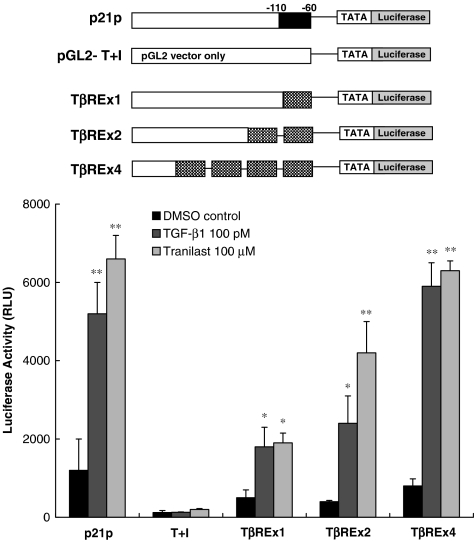
The sequence between −74 and −83 bp was sufficient for both TGF-β- and Tranilast-mediated activation of p21 promoter
As shown in Figure 4, pGL2-T+I, a basic promoter containing TATA box and initiator, was unresponsive to both TGF-β1 and Tranilast. The insertion of one, two, or four copies of the sequence between −74 and −83 bp (TβRE) conferred a level of responsiveness to both TGF-β1 and Tranilast that was proportional to the number of copies inserted. The insertion of four copies resulted in responsiveness comparable to that elicited by constructs of the full-length p21 promoter p21P. This result suggests that this sequence is sufficient for both the TGF-β- and Tranilast-mediated activation of the p21 promoter.
Figure 4.
The sequence between −74 and −83 is sufficient to mediate both TGF-β1 and Tranilast-mediated activation. pGL2-T+I, a basic promoter containing a TATA box and initiator, was unresponsive to both TGF-β1 and Tranilast. The insertion of one, two, or four copies of the sequence between −74 and −83 bp (TβRE) conferred similar responsiveness to TGF-β1 and Tranilast. Each value represents the mean±s.d. (n=6). *P<0.05; **P<0.01 as compared to DMSO control in each group.
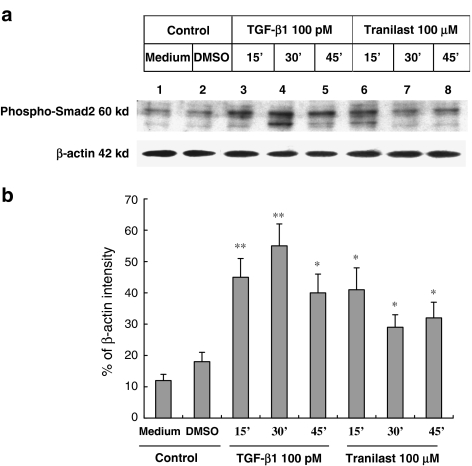
Tranilast induced the activation of Smad2
Smad2 is a cytoplasmic mediator of the TGF-β signaling pathway. TGF-β induces the phosphorylation of Smad2 by activating the TGF-β type I receptor, a serine and threonine kinase receptor. To determine the effect of Tranilast on the phosphorylation status of Smad2, HaCaT cells were treated with either TGF-β or Tranilast and then the increase in phospho-Smad2 was determined by Western blot using anti-phospho-Smad2 antibody. As shown in Figure 5, the phosphorylation of Smad2 in HaCaT cells treated with 100 pM TGF-β or 100 μM Tranilast increased at 15, 30 and 45 min after drug treatment, suggesting that Tranilast may share the same signaling pathway of TGF-β.
Figure 5.
Activation of Smad2 by Tranilast. (a) HaCaT cells were treated with 100 pmol l−1 (pM) TGF-β1 (lanes 3, 4 and 5) or 100 μmol l−1 (μM) Tranilast (lanes 6, 7 and 8). The cells were harvested every 15 min for protein analysis. Total proteins from cell lysates were separated and probed with anti-phospho-Smad2 antibody. Phospho-Smad2 is a 60-kDa protein. The β-actin was also probed to demonstrate the equal loading of protein for each group. (b) Quantitative analysis with β-actin normalization showed significant stimulatory effects of TGF-β1 and Tranilast on protein expression level of phospho-Smad2. Each experiment was repeated three times. *P<0.05; **P<0.01 as compared to medium control in each group.
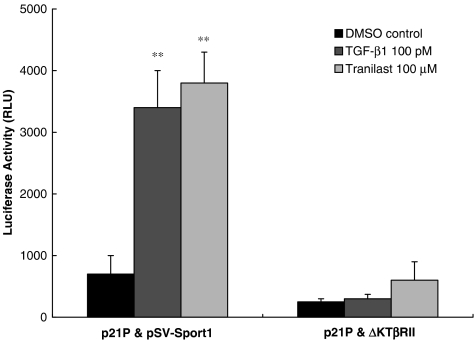
ΔkTβRII blocks both Tranilast and TGFβ signaling
In HaCaT cells cotransfected with p21P and ΔkTβRII (a truncated form of TGF-β type II receptor cloned into pSV-Sport1) followed by Tranilast or TGF-β treatment, luciferase activity was markedly reduced when compared with luciferase activity in cells cotransfected with p21P and pSV-Sport1 (vector control). Both the Tranilast- and TGF-β-mediated responses were suppressed to a similar extent in cells cotransfected with the truncated version of TβRII. These findings suggest that the initial step in both Tranilast- and TGF-β-mediated signaling is through interaction with TβRII (Figure 6).
Figure 6.
ΔkTβRII blocks both Tranilast and TGF-β signaling. HaCaT cells were cotransfected with p21P and ΔkTβRII, both cloned into pSV-Sport1 (or p21P and pSV-Sport1 as vector control) and then treated with 100 μmol l−1 ( μM) Tranilast or 100 pmol l (pM) TGF-β1. Cotransfection with ΔkTβRII resulted in marked and similar reduction in luciferase activity of Tranilast- and TGF-β1-stimulated cells, implying TβRII is needed for both Tranilast- and TGF-β1-mediated signaling. Each value represents the mean±s.d. (n=6). **P<0.01 as compared to medium control in each group.
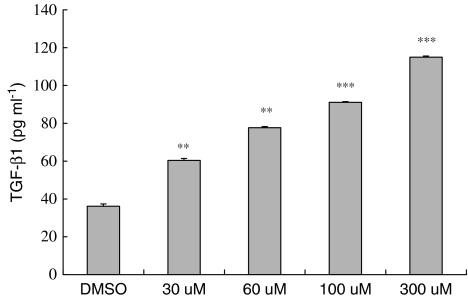
Tranilast increases TGF-β level
To further elucidate the relationship between Tranilast and TGF-β, HaCaT cells treated with increasing doses of Tranilast (30, 60, 100, and 300 μM) were subjected to TGF-β level measurement. After correction with total protein concentration, the TGF-β level was significantly increased by Tranilast in a dose-dependent manner (Figure 7).
Figure 7.
Activation of Tranilast on TGF-β1 level. HaCaT cells were treated with 30, 60, 100, and 300 μM of Tranilast with DMSO as the control. The secretion level of TGF-β was corrected by total protein from each treated group. Tranilast induced TGF-β secretion from HaCaT cells in a dose-dependent manner. Each value represents the mean±s.d. (n=4). **P<0.01, ***P<0.001 as compared to DMSO control in each group.
Discussion
Tranilast has shown promise in preventing restenosis, a serious long-term complication after balloon angioplasty. Tranilast is believed to act by inhibiting the proliferation and migration of vascular SMCs, likely through induction of p21 and p53. This in vitro study examined the underlying molecular mechanism leading to the induction of p21 by Tranilast.
Using deletion mutagenesis and luciferase activity as a surrogate measurement of p21 transcriptional activity, we have identified a region between −74 and −83 bp of the p21 promoter as a region that is responsive to Tranilast in HaCaT cells. This same region on the p21 promoter has been previously identified as a TGF-β-responsive element (Datto et al., 1995b). TGF-β has been shown to elicit rapid transcriptional activation of p21 thereby arresting cell growth in the G1 phase of the cell cycle (Datto et al., 1995a). In Figure 3, we found that the mutant #2 with deletion mutagenesis between −74 and −83 bp completely blocked the luciferase activity of p21 promoter. Although the mutants #3 and #4 had a reduced ability to be activated by TGF-β and Tranilast, the luciferase activity can still be substantially induced 40% to 2.5-fold by TGF-β and Tranilast as compared to the DMSO control. This result suggested that the region between −54 and −73 was involved but not fully responsible for the p21 promoter activation.
To confirm that both Tranilast and TGF-β do in fact act at the same promoter site, we transfected HaCaT cells with the −74/−83 bp of the p21 promoter region, and showed that both the Tranilast- and TGF-β-mediated luciferase activity increased in parallel and was of similar magnitude. When multiple copies of the −74/−83 bp responsive element were inserted, both Tranilast- and TGF-β-mediated luciferase activities increased in proportion to the number of copies of responsive element per plasmid present. Maximal luciferase activity was attained with the four-copy construct, which was approximately equal to that attained with the full-length p21 construct. The −74/−83 bp responsive element therefore was sufficient to induce p21 transcription by both Tranilast- and TGF-β. It was of interest to notice that the TβREx1 construct containing one copy of TβRE exerted less potent luciferase activity as compared to the full-length p21 construct. In addition to the TβRE located at the region between −74 and −83, there may be some other regions of the p21 promoter regulating the TβRE activity. That is why TβREx1 alone exerted less potent transcriptional activity as compared to the p21P in response to TGF-β and Tranilast treatment. Although mutation in TβRE may result in a significant loss of the p21 promoter activity stimulated by TGF-β and Tranilast, yet expression of only one copy of TβRE in luciferase vector was not able to express full promoter activity.
As both Tranilast and TGF-β induce p21 expression by acting at a common site on the promoter, we postulated that both Tranilast and TGF-β might share a common signaling pathway, namely the TGF-β signaling pathway. To decipher this, we looked at Smad2 phosphorylation. Smad2 is a cytoplasmic mediator of the TGF-β signaling pathway and TGF-β induces its phosphorylation. We observed that both Tranilast and TGF-β produced parallel increases in phosphorylated Smad (Figure 5). As Smad2 phosphorylation by TGF-β is mediated via the TGF-β type II receptor, a serine/threonine kinase receptor (Fiocchi, 2001), we postulated that Tranilast, like TGF-β, might act at the receptor level, mediating either an increase in the number of TGF-β receptors and/or in the kinase activity of existing TGF-β receptors. To demonstrate this, we expressed a ΔkTβRII that is known to exert a dominant-negative effect on TGF-β signaling in HaCaT cells and showed that these cells were now no longer responsive to either Tranilast or TGF-β. Similarly, blocking TGFβRII by ΔkTβRII can then be expected to downregulate the phosphorylated-Smad2 level when stimulated with TGF-β and Tranilast. Therefore, our results indicate that Tranilast- or TGF-β-mediated p21 induction requires the presence of an intact TGF-β type II receptor. We conclude from the foregoing that Tranilast likely acts via the TGF-β signaling pathway. This is further confirmed by our finding that TGF-β was significantly increased by Tranilast in a dose-dependent manner (Figure 7). These data can provide a related evidence that Tranilast activates p21 gene expression via the TGF-β-mediated signaling pathway.
In direct contrast to our results, Ward et al. (2002) in two in vivo models, a rat model of balloon angioplasty and a Boston minipig model of angioplasty with stenting, have reported that TGF-β and Tranilast antagonized each other, with Tranilast inhibiting TGF-β-mediated SMC proliferation. They further suggested that inhibition of restenosis by Tranilast was due to suppression of the injury-induced rise in TGF-β, TGF-β receptors, and integrins αν and β3 levels (Ward et al., 1998; 2002). This apparent conflict between Ward and colleagues' conclusions and ours might be explained by the fact that we used a drastically different cell model, an in vitro semiconfluent human keratinocyte (HaCaT) monolayer, from theirs. Whether TGF-β stimulates or inhibits cell proliferation is dependent on the cell type (e.g., fibroblast or keratinocyte) in the healing wound (Ashcroft et al., 1999).
The density of the cell monolayer might also contribute to the differences in response to TGF-β. Majack et al. and Li et al., 1995 showed that proliferation of confluent SMCs was stimulated whereas that of nonconfluent SMCs was inhibited by TGF-β (Majack, 1987; Goodman & Majack, 1989). In addition, the activity of Smad proteins (inhibition or stimulation of translation) depends on the mixture of coactivators and/or corepressors with which they complex, and since TGF-β may stimulate or inhibit cell proliferation directly or through activation of Smads, the outcome is highly dependent on the cellular activity of TGF-β (Massague & Wotton, 2000). Although we have proposed a common pathway for Tranilast- and TGF-β in this study, this may only apply to inhibition of SMC proliferation. During fibrotic tissue response to angioplasty, for instance, TGF-β was found to stimulate and Tranilast to inhibit collagen and glucosaminoglycan deposition (Miyazawa et al., 1995).
The implication of our present study is that the effect of Tranilast or other drugs that act via the TGF-β-responsive element might be potentially enhanced by the increased level of TGF-β-responsive element and/or TGF-β receptors at the site of angioplasty or stenting through genetical engineering. Additionally, the effects of Tranilast could be amplified by concurrent use of drugs that inhibit SMC proliferation by a different mechanism. For example, Tranilast combined with inhibitors of farnesyl transferase (farnesyl pyrophosphate analogues), an enzyme that added a farnesyl moiety onto the carboxy terminus of H-ras responsible for membrane adherence, was found to prevent restenosis after balloon angioplasty in pig coronary artery (Work et al., 2001).
It should be borne in mind, however, that our study is limited to a single set of in vitro conditions that showed TGF-β and Tranilast inhibit HaCaT cell proliferation via the TGF-β-responsive element. In addition, the rationale for using keratinocyte HaCaT cell line instead of SMC in our present study is due to extensive reports of the TGF-β effects on the functional analysis of p21 promoter activity being conducted in this cell line (Datto et al., 1995a, 1995b). We therefore used this cell line to ensure that the functional activities of p21 promoter can be induced by TGF-β in a reproducible manner to evaluate the transcriptional effects of Tranilast on p21 promoter. A similar in vivo comparison of the effects of TGF-β and Tranilast on SMC proliferation is necessary to confirm the in vivo and clinical relevance of our finding.
In conclusion, the sequence between −74 and −83 bp of p21 promoter, previously identified as TGF-β-response element, is demonstrated here to be sufficient for the transcriptional activation of p21 by Tranilast. Furthermore, Tranilast can induce the phosphorylation of Smad2, a cytoplasmic signaling molecule essential for mediating TGF-β signal transduction, suggesting that the induction of p21 expression by Tranilast might be mediated through the activation of the TGF-β signal transduction pathway.
Acknowledgments
We acknowledge the valuable technical assistance provided by Ms Shu-Chuan Lin. This work was supported by a grant from the National Science Council (NSC-90-2314-B-075-048; NSC91-2745-B-039-001; DMR90-136).
Abbreviations
- ΔkTβRII
truncated form of TGF-β type II receptor
- CDK
cyclin-dependent kinase
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- F12
Ham's nutrient mixture F-12
- HRP
horse-radish-peroxidase
- LDH
lactate dehydorgenase
- MTT, 3-[4,5-dimethylthiazol-2-yl]-2
5-diphenyl tetrazolium bromide
- PBS
phosphate-buffered saline
- PDGF
platelet-derived growth factor
- PTCA
percutaneous transluminal coronary angioplasty
- SMCs
smooth muscle cells
- TGF-β
transforming growth factor-β
- TREAT-2
the second Tranilast REstenosis following Angioplasty Trial
- TβRE
TGF-β response element
References
- ASHCROFT G.S., YANG X., GLICK A.B., WEINSTEIN M., LETTERIO J.L., MIZEL D.E., ANZANO M., GREENWELL-WILD T., WAHL S.M., DENG C., ROBERTS A.B. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- BOUKAMP P., PETRUSSEVSKA R.T., BREITKREUTZ D., HORNUNG J., MARKHAM A., FUSENIG N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAND T., MACLELLAN W.R., SCHNEIDER M.D. A dominant-negative receptor for type beta transforming growth factors created by deletion of the kinase domain. J. Biol. Chem. 1993;268:11500–11503. [PubMed] [Google Scholar]
- CHARNG M.J., KINNUNEN P., HAWKER J., BRAND T., SCHNEIDER M.D. FKBP-12 recognition is dispensable for signal generation by type I transforming growth factor-beta receptors. J. Biol. Chem. 1996;271:22941–22944. doi: 10.1074/jbc.271.38.22941. [DOI] [PubMed] [Google Scholar]
- DATTO M.B., LI Y., PANUS J.F., HOWE D.J., XIONG Y., WANG X.F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl. Acad. Sci. U.S.A. 1995a;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTO M.B., YU Y., WANG X.F. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J. Biol. Chem. 1995b;270:28623–28628. doi: 10.1074/jbc.270.48.28623. [DOI] [PubMed] [Google Scholar]
- FIOCCHI C. TGF-beta/Smad signaling defects in inflammatory bowel disease: mechanisms and possible novel therapies for chronic inflammation. J. Clin. Invest. 2001;108:523–526. doi: 10.1172/JCI13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHMAN D.L., LEON M.B., BAIM D.S., SCHATZ R.A., SAVAGE M.P., PENN I., DETRE K., VELTRI L., RICCI D., NOBUYOSHI M., CLEMAN M., HEUSER R., ALMOND D., TEIRSTEIN P.S., FISH R.D., COLOMBO A., BRINKER J., MOSES J., SHAKNOVICH A., HIRSHFELD J., BAILEY S., ELLIS S., RAKE R., GOLDBERG S. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- FRANKLIN S.M., FAXON D.P. Pharmacologic prevention of restenosis after coronary angioplasty: review of the randomized clinical trials. Coron. Artery Dis. 1993;4:232–242. doi: 10.1097/00019501-199303000-00003. [DOI] [PubMed] [Google Scholar]
- FUKUYAMA J., MIYAZAWA K., HAMANO S., UJIIE A. Inhibitory effects of tranilast on proliferation, migration, and collagen synthesis of human vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1996;74:80–84. [PubMed] [Google Scholar]
- GOODMAN L.V., MAJACK R.A. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J. Biol. Chem. 1989;264:5241–5244. [PubMed] [Google Scholar]
- KOSUGA K., TAMAI H., UEDA K., HSU Y.S., ONO S., TANAKA S., DOI T., MYOU U.W., MOTOHARA S., UEHATA H. Effectiveness of tranilast on restenosis after directional coronary atherectomy. Am. Heart J. 1997;134:712–718. doi: 10.1016/s0002-8703(97)70055-3. [DOI] [PubMed] [Google Scholar]
- KUSAMA H., KIKUCHI S., TAZAWA S., KATSUNO K., BABA Y., ZHAI Y.L., NIKAIDO T., FUJII S. Tranilast inhibits the proliferation of human coronary smooth muscle cell through the activation of p21waf1. Atherosclerosis. 1999;143:307–313. doi: 10.1016/s0021-9150(98)00308-6. [DOI] [PubMed] [Google Scholar]
- LI Z., MOORE S., ALAVI M.Z. Mitogenic factors released from smooth muscle cells are responsible for neointimal cell proliferation after balloon catheter deendothelialization. Exp. Mol. Pathol. 1995;63:77–86. doi: 10.1006/exmp.1995.1032. [DOI] [PubMed] [Google Scholar]
- MAJACK R.A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J. Cell Biol. 1987;105:465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSAGUE J., WOTTON D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAZAWA K., KIKUCHI S., FUKUYAMA J., HAMANO S., UJIIE A. Inhibition of PDGF- and TGF-beta 1-induced collagen synthesis, migration and proliferation by tranilast in vascular smooth muscle cells from spontaneously hypertensive rats. Atherosclerosis. 1995;118:213–221. doi: 10.1016/0021-9150(95)05607-6. [DOI] [PubMed] [Google Scholar]
- NOBUYOSHI M., KIMURA T., NOSAKA H., MIOKA S., UENO K., YOKOI H., HAMASAKI N., HORIUCHI H., OHISHI H. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J. Am. Coll. Cardiol. 1988;12:616–623. doi: 10.1016/s0735-1097(88)80046-9. [DOI] [PubMed] [Google Scholar]
- ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- SAVAGE M.P., FISCHMAN D.L., RAKE R., LEON M.B., SCHATZ R.A., PENN I., NOBUYOSHI M., MOSES J., HIRSHFELD J., HEUSER R., BAIM D., CLEMAN M., BRINKER J., GEBHARDT S., GOLDBERG S. Efficacy of coronary stenting versus balloon angioplasty in small coronary arteries. Stent Restenosis Study (STRESS) Investigators. J. Am. Coll. Cardiol. 1998;31:307–311. doi: 10.1016/s0735-1097(97)00511-1. [DOI] [PubMed] [Google Scholar]
- SERRUYS P.W., DE JAEGERE P., KIEMENEIJ F., MACAYA C., RUTSCH W., HEYNDRICKX G., EMANUELSSON H., MARCO J., LEGRAND V., MATERNE P., BELARDI J., SIGWART U., COLOMBO A., JACQUES GOY J., VAN DEN HEUVEL P., DELCAN J., MOREL M. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI A., TANIGUCHI T., ISHIKAWA Y., YOKOYAMA M. Tranilast inhibits vascular smooth muscle cell growth and intimal hyperplasia by induction of p21(waf1/cip1/sdi1) and p53. Circ. Res. 1999;84:543–550. doi: 10.1161/01.res.84.5.543. [DOI] [PubMed] [Google Scholar]
- TAMAI H., KATOH K., YAMAGUCHI T., HAYAKAWA H., KANMATSUSE K., HAZE K., AIZAWA T., NAKANISHI S., SUZUKI S., SUZUKI T., TAKASE S., NISHIKAWA H., KATOH O. The impact of tranilast on restenosis after coronary angioplasty: the Second Tranilast Restenosis Following Angioplasty Trial (TREAT-2) Am. Heart J. 2002;143:506–513. doi: 10.1067/mhj.2002.120770. [DOI] [PubMed] [Google Scholar]
- WARD M.R., AGROTIS A., KANELLAKIS P., HALL J., JENNINGS G., BOBIK A. Tranilast prevents activation of transforming growth factor-beta system, leukocyte accumulation, and neointimal growth in porcine coronary arteries after stenting. Arterioscler. Thromb. Vasc. Biol. 2002;22:940–948. doi: 10.1161/01.atv.0000019405.84384.9c. [DOI] [PubMed] [Google Scholar]
- WARD M.R., SASAHARA T., AGROTIS A., DILLEY R.J., JENNINGS G.L., BOBIK A. Inhibitory effects of tranilast on expression of transforming growth factor-beta isoforms and receptors in injured arteries. Atherosclerosis. 1998;137:267–275. doi: 10.1016/s0021-9150(97)00275-x. [DOI] [PubMed] [Google Scholar]
- WORK L.M., MCPHADEN A.R., PYNE N.J., PYNE S., WADSWORTH R.M., WAINWRIGHT C.L. Short-term local delivery of an inhibitor of Ras farnesyltransferase prevents neointima formation in vivo after porcine coronary balloon angioplasty. Circulation. 2001;104:1538–1543. doi: 10.1161/hc3801.095661. [DOI] [PubMed] [Google Scholar]