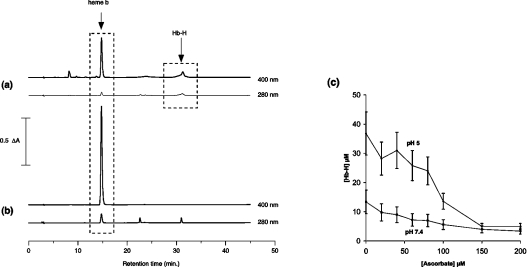
Figure 7. Effects of ascorbate on peroxidative damage to haemoglobin.
Reverse-phase HPLC chromatogram of metHb–DBBF (100 μM) reacted with H2O2 (200 μM) for 1 h at 37 °C in sodium acetate (20 mM), pH 5.0, (a) in the absence and (b) in the presence of 0.6 mM sodium ascorbate. The peak at 15 min is free haem b (visible at both 400 nm and 280 nm detection). The peaks at 22.7 and 31 min are from the β- and modified α-chains in Hb–DBBF. Normally, these are only detectable at 280 nm, but, in the absence of ascorbate, there is significant absorbance at 400 nm, indicating a covalent haem:protein attachment that cannot be disrupted at the pH of the HPLC column (pH 2). The optical spectrum of this species (not shown) is that previously ascribed to this covalent haem:protein species following peroxide addition to HbAo (termed Hb-H). (c) Effect of varying the ascorbate concentration on the formation of Hb-H at pH 5.0 (in 20 mM sodium acetate) and pH 7.4 (in 20 mM sodium phosphate) at 37 °C.