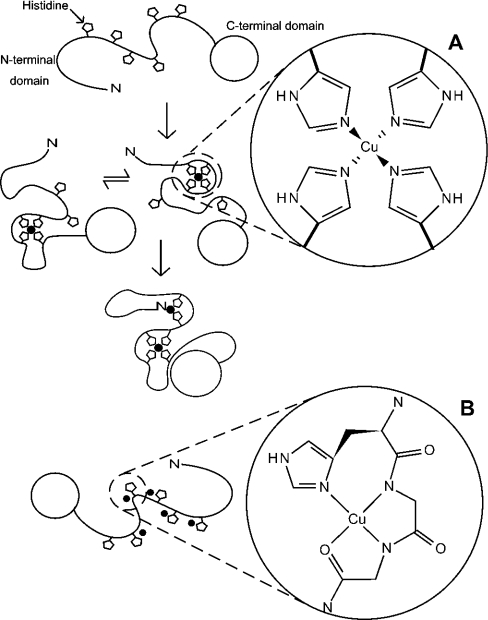
Figure 7. An illustration of the two alternative modes of Cu2+ co-ordination in the full-length prion protein with details showing the co-ordination environment of the Cu2+ ion in each case.
Binding of Cu2+ by the full-length prion protein at pH 5.5 involves co-ordination by an exchanging combination of histidine imidazoles (A). The Figure shows apo-PrP (top), the PrP–Cu2+ ensemble (middle) and PrP–2Cu2+ (bottom), which may also be an ensemble of different co-ordinations involving the same ligands. Note that the assignment of particular combinations of ligands to any one complex or metal centre is arbitrary. The histidine Nϵ co-ordination shown is based on an IR study of multiple histidine co-ordination in the octapeptide repeats [13]. At pH 7.4, with saturating concentrations of copper, the full-length prion protein can bind at least five Cu2+ ions, each co-ordinated by a single imidazole and nearby backbone amides (B).