Abstract
Despite theoretical predictions, there is little empirical evidence that kin competition avoidance promotes dispersal. We show that dispersal by male Platyscapa awekei pollinating fig wasps is promoted by both low returns in the natal fig and kin competition avoidance, with strategies depending on the interaction between phenotype (body size) and local conditions. We discuss the paucity of similar work, how males might assess conditions, and then contrast male dispersal and fighting behaviour. This indicates that differences in the scale at which behaviours affect competition can mean that they are the product of dissimilar selective forces even when they have the same recipients. More generally, this could explain why other social interactions are often mixtures of cooperation and conflict.
Keywords: dispersal, kin competition, fig wasps
1. Introduction
Dispersal is common in nature. It is hypothesized to be promoted by: (i) low returns due to limited resource availability and/or intraspecific competition, (ii) costs to inbreeding and (iii) inclusive fitness benefits due to reduced kin competition (see Clobert et al. 2001). However, empirical testing is often difficult: multiple factors may promote dispersal, and strategies may also depend on phenotype and/or local conditions (see Ims & Hjermann 2001). In particular, demonstrations of kin competition avoidance are rare, with investigations often confounded because dispersal should also increase with relatedness if promoted by costs to inbreeding.
Since they are inbred haplodiploids and unlikely to suffer inbreeding costs (Werren 1993; but see Henter 2003), this problem does not arise in male pollinating fig wasps. Wasp natural history is as follows: a few females (foundresses) enter each fig and gall and oviposit in the flowers inside. The larvae mature, then the males search for females, excavate holes into their galls and mate them, and excavate a tunnel through the fig wall so that they can exit. Males of most species then die, but in some they go on to mate females in other figs (Greeff et al. 2003). Males do not disperse only after fully exploiting the fig: in Platyscapa awekei, pollinator of Ficus salicifolia, a mean±s.d. of only 34±26% (range=0–89%) of females are mated at exit tunnel completion (A. Loggenberg 2003, unpublished data). We made several predictions, assuming strategies depend on local conditions. First, if promoted by low returns we predicted that dispersal should decrease with female number (resource availability) and increase with male number (intraspecific competition) in the fig. Second, if promoted by kin competition avoidance we predicted that dispersal should decrease with foundress density (decreasing relatedness). Third, we predicted a phenotypic effect: males often fight for mates, so assuming large males win more often, dispersal should decrease with body size.
We tested these predictions in P. awekei, whose males both disperse and fight non-lethally for mates (Greeff et al. 2003).
2. Material and Methods
We investigated the factors affecting dispersal by introducing foundresses into figs on F. salicifolia trees on the University of Pretoria campus, South Africa (25°45′ S 28°14′ E) that had previously been bagged to prevent oviposition (see Moore et al. 2002 for methods). We left the figs until just before wasp release, then placed them in mesh-lidded pots. Forty-eight hours later, we counted the females and the (dispersing) males in the pots (figs are not re-entered: J. C. Moore 2003, personal observation) and estimated male body sizes by measuring left hind tibia lengths. Adult males live for a mean±s.d. of 20±9 h (R. Nelson 2003, unpublished data), so most dispersers would have left by this time. We then opened the figs, counted any (philopatric) males inside and measured them. We performed 41 single and 20 two-foundress (in which each originated from a different fig) experiments from November 2002 to January 2003, and obtained data on 371 males. We analysed the data using a Generalized Linear Mixed Model (GLMM) with binomial errors (library glmmPQL from Venables & Ripley 2002) imported into Splus v.6 Professional Edition (2001, Insightful Corp, Seattle, WA). We scored each male as philopatric (0) or dispersing (1), then fitted a model with foundress density as a fixed factor, natal fig as a random factor, male number, female number and body size (tibia length cubed) as covariates, and all interactions.
3. Results
In the experiment, brood size and female number did not differ between foundress densities (T-tests with unequal variances, T25.23=−0.75, NS; mean±s.d.=39.84±13.74, range=16–85 and T26.57=−0.12, NS; mean±s.d.=33.44±11.74, range=13–75, respectively). However, there was a marginally significant increase in male number with density (data square root transformed: T23.65=−1.88, p=0.07; single-foundress back-transformed mean=5.21, 95% CI=4.47–5.98, range=2–12; two-foundress mean=7.41, 95% CI=5.04–10.23, range=2–19), probably due to the influence of local mate competition on foundress sex ratios (see Herre 1985). Overall, 64.1% of males dispersed from single-foundress figs and 70.6% from two-foundress figs. The GLMM indicated a four-way interaction between explanatory variables (F1,297=9.68, p<0.01), so we analysed the data at each foundress density separately. In single-foundress figs, an interaction between female number, male number and body size existed (F1,170=9.10, p<0.01). Plotting model predictions indicated that at low male number dispersal decreased with female number (the magnitude increased with size), and decreased with size (figure 1a). Increasing male number had mixed effects. Small male dispersal declined more sharply with female number than before, so it increased when female number was low but began to decrease at higher number. Larger male dispersal also mostly increased, with the relationship with female number becoming positive. Dispersal continued to decrease with size when female number was low, but as numbers increased the effect declined and reversed, and it began to increase with size.
Figure 1.
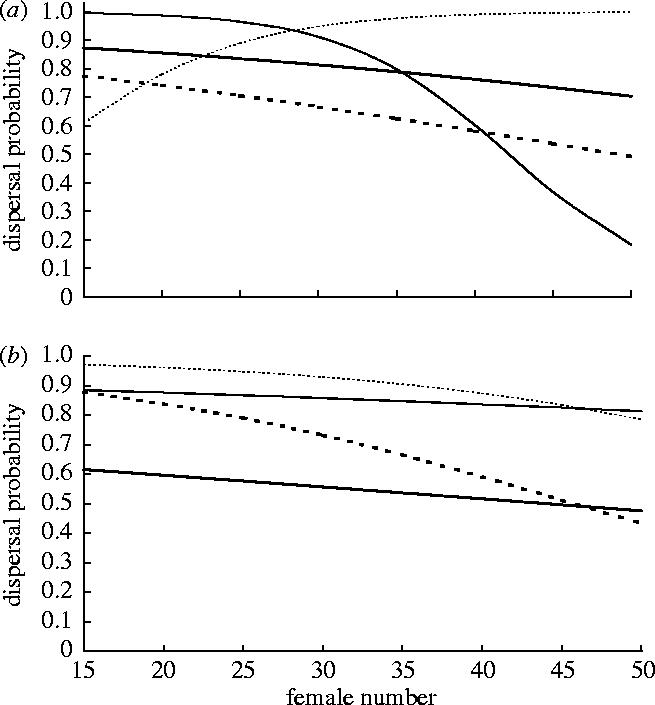
Relationships between female number and predicted dispersal probability in: (a) single-foundress and (b) two-foundress figs. Thick lines represent predictions when male number is low (=6), thin lines predictions when male number is high (=12). Solid lines represent predictions for small males (tibia length3=0.004 mm3), dotted lines predictions for large males (tibia length3=0.012 mm3).
In two-foundress figs, there was an interaction between female number and size (F1,129=5.16, p<0.05) and a marginal male number effect (F1,17=3.24, p=0.09; other interactions p>0.45). Dispersal decreased with female number (again the magnitude increased with size), increased with male number, and almost always decreased with size (figure 1b). Foundress density effects can be discerned by comparing similar lines in figures 1a and 1b. When male number was low, small male dispersal decreased considerably with density. However, this effect declined and eventually reversed as size increased. As male number increased, effects also began to depend on female number, with small males eventually dispersing more from single-foundress figs when it was low and from two-foundress figs when it was high. The opposing changes were observed in larger males.
4. Discussion
Our results indicate that P. awekei strategies depend on conditions in the natal fig, but they were not always consistent with the predictions made about the factors promoting dispersal. As predicted if promoted by low returns, dispersal generally decreased with female number (resource availability) and increased with male number (intraspecific competition) in the fig. However, female number effects increased with body size, and sometimes in single-foundress figs the opposite relationships with female and male number were observed. Similarly, as predicted if promoted by kin competition avoidance, at low male number dispersal by most males decreased with foundress density (decreasing relatedness). However, dispersal by large males increased slightly, and as male number increased effects began to depend on both size and female number. Also, as predicted given that large males will win more fights for mates, dispersal often decreased with size. However, at other times it increased. In short, factor effects were often phenotype- (body size) and context- (in terms of levels of other factors) dependent.
We suggest the reason for this is that factors interact with each other and with male phenotype to determine returns from the fig. Due to their effect on competitor encounter rates, theory predicts that the ability of large males to monopolize mates, and therefore the relationship between body size and mating success, will depend on female and male number in the fig (Murray 1987). Increasing female number will increase this ability by decreasing encounters, which is probably why female number effects increased with size: larger males tend to obtain the extra mates. Increasing male number will decrease it by increasing encounters. When coupled with the inclusive fitness benefits of dispersal, this probably explains strategies in single-foundress figs. At low male number, large males are able to monopolize mates and tend to remain philopatric, so smaller males disperse. At higher male number this ability declines, so large males (who can expect high returns because they will also be large in other figs) tend to disperse and smaller males remain philopatric to take advantage of the increased mating opportunities. The role of kin competition avoidance in these adjustments is confirmed by different patterns observed in two-foundress figs. The latter, though, are likely to be the result of a similar male number effect, caused by the strategy changes of other phenotypes: increases in large male dispersal compared with single-foundress figs and in dispersal with size are probably due to increased smaller male mating opportunities. Work investigating the factors influencing male mating success is now underway to fully test our arguments.
From these findings, it is clear that low returns and kin competition avoidance combine to promote P. awekei male dispersal. This is a rare demonstration of kin competition avoidance promoting dispersal. We reserve comment on whether this paucity reflects nature. Investigations can be confounded because predictions are similar when costs to inbreeding promote dispersal (we avoided this problem because fig wasps are unlikely to suffer from inbreeding depression). Also, as our work shows effects can be difficult to distinguish because strategies may be phenotype dependent and/or multiple promoting factors may interact. This has been recorded in other species (see Ims & Hjermann 2001), but we posit the mechanism involved: the dependence of the relationship between body size and mating success on resource availability and intraspecific competition. Given this mechanism, our findings further imply that strategies can be affected by those of other phenotypes, even to the point where adjustments seem counter to hypothesized promoting factors. Similar is probably true in other species (see for example, the body size-dependent response to mother–daughter competition in the reptile Lacerta viviparta found by Le Galliard et al. 2003). Therefore, more studies such as ours are required to assess how common kin competition avoidance really is. In P. awekei, work should now also concentrate on how strategies are adjusted, which is rarely known in any species. We suggest that it is to an extent facultative, with encounter rates used to assess male and female number. Adjustment to kin competition levels, though, could be a maternal effect, as in some non-pollinating fig wasps where offspring dispersal morph is determined after assessing foundress density (Pienaar & Greeff 2003). This could be tested by irradiating foundresses so that their eggs do not develop (see Kinoshita et al. 2002): dispersal from figs oviposited in by irradiated foundresses similar to that from figs oviposited in by only normal foundresses would indicate a maternal effect.
Additionally, our findings have implications for social evolution theory. We have shown that kin selection can promote male fig wasp dispersal. However, comparative work on these species indicates that it does not similarly promote reduced aggression towards siblings in fights for mates (West et al. 2001). This difference probably occurs because the former behaviour only increases competition at a global scale, whereas the confined fig environment means that selection for the latter is cancelled out by the effects of increased competition among relatives (see West et al. 2002; it should be noted, though, that such local competition could lead to selection for spiteful behaviour towards negatively related competitors: see Gardner & West 2004). It demonstrates how differences in the scale at which behaviours affect competition can mean that they are the product of dissimilar selective forces even when they have the same recipients, and therefore provides new evidence of the role of the scale of competition in shaping social behaviour, alongside the aforementioned comparative study (West et al. 2001) and recent experimental work (Gardner et al. 2004; Griffin et al. 2004). Investigations should now focus on whether similar differences can explain the mixtures of conflict and cooperation often observed in other social interactions.
In summary, we have shown that low returns and kin competition avoidance promote P. awekei male dispersal, with strategies depending on the interaction between phenotype (body size) and local conditions. We have discussed the paucity of similar studies, how males assess conditions, and how differences in the scale at which the two behaviours affect competition mean that strategies in fights for mates will be determined by dissimilar selective forces even though they involve the same recipients. More generally, differences of this type may explain why social interactions are often mixtures of cooperation and conflict.
Acknowledgments
This material is based upon work supported by the National Research Foundation under grant no. 2053809 to J.M.G. Any opinion, findings and conclusions or recommendations expressed in this material do not necessarily reflect the views of the National Research Foundation.
References
- Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Gardner A, West S.A. Spite and the scale of competition. J. Evol. Biol. 2004;17:1195–1203. doi: 10.1111/j.1420-9101.2004.00775.x. doi:10.1111/j.1420-9101.2004.00775.x [DOI] [PubMed] [Google Scholar]
- Gardner A, West S.A, Buckling A. Bacteriocins, spite and virulence. Proc. R. Soc. B. 2004;271:1529–1535. doi: 10.1098/rspb.2004.2756. doi:10.1098/rspb.2004.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeff J.M, Van Noort S, Rasplus J.-Y, Kjellberg K. Dispersal and fighting in male pollinating fig wasps. C. R. Biol. 2003;326:121–130. doi: 10.1016/s1631-0691(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Griffin A.S, West S.A, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. doi:10.1038/nature02744 [DOI] [PubMed] [Google Scholar]
- Henter H.J. Inbreeding depression and haplodiploidy: experimental measures in a parasitoid and comparisons across diploid and haplodiploid insect taxa. Evolution. 2003;57:1793–1803. doi: 10.1111/j.0014-3820.2003.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Herre E.A. Sex ratio adjustment in fig wasps. Science. 1985;228:896–898. doi: 10.1126/science.228.4701.896. [DOI] [PubMed] [Google Scholar]
- Ims R.A, Hjermann D.O. Condition-dependent dispersal. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; Oxford: 2001. p. 216. [Google Scholar]
- Kinoshita M, Kasuya E, Yahara T. Effects of time-dependent competition for oviposition sites on clutch sizes and sex ratios to in a fig wasp. Oikos. 2002;96:31–35. doi:10.1034/j.1600-0706.2002.960103.x [Google Scholar]
- Le Galliard J.-F, Ferriere R, Clobert J. Mother–offspring interactions affect natal dispersal in a lizard. Proc. R. Soc. B. 2003;270:1163–1169. doi: 10.1098/rspb.2003.2360. doi:10.1098/rspb.2003.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.C, Compton S.G, Hatcher M.J, Dunn A.D. Quantitative tests of sex ratio models in a pollinating fig wasp. Anim. Behav. 2002;64:23–32. doi:10.1006/anbe.2002.3034 [Google Scholar]
- Murray M.G. The closed environment of the fig receptacle and its influence on male conflict in the Old World fig wasp. Philotrypesis pilosa. Anim. Behav. 1987;35:488–506. [Google Scholar]
- Pienaar J, Greeff J.M. Maternal control of offspring sex and male morphology in the Otitesella fig wasps. J. Evol. Biol. 2003;16:244–253. doi: 10.1046/j.1420-9101.2003.00522.x. doi:10.1046/j.1420-9101.2003.00522.x [DOI] [PubMed] [Google Scholar]
- Venables W.N, Ripley B.D. 4th edn. Springer; Berlin: 2002. Modern applied statistics with S. [Google Scholar]
- Werren J.H. The evolution of inbreeding in haplodiploid organisms. In: Thornhill N.W, Shields W.M, editors. The natural history of inbreeding and outbreeding: theoretical and empirical perspectives. University of Chicago Press; Illinois: 1993. pp. 42–59. [Google Scholar]
- West S.A, Murray M.G, Machado C.A, Griffin A.S, Herre E.A. Testing Hamilton's rule with competition between relatives. Nature. 2001;409:510–513. doi: 10.1038/35054057. doi:10.1038/35054057 [DOI] [PubMed] [Google Scholar]
- West S.A, Pen I, Griffin A.S. Cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]


