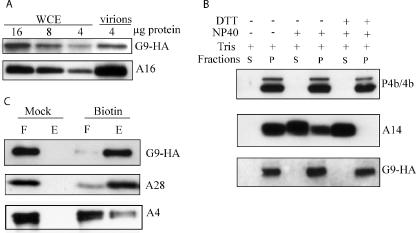
FIG. 3.
Association of G9 protein with purified virions. (A) Whole-cell extracts (WCE) or sucrose gradient-purified virions from cells infected with vG9i in the presence of IPTG were analyzed by SDS-PAGE and Western blotting using anti-HA MAb. The amounts of total protein (4 to 16 μg) applied to the gel are indicated. (B) Extraction of G9 from purified virions. Virions prepared as in panel A were treated with 1% NP-40 with or without 50 mM DTT for 1 h at 37°C and separated into soluble (S) or insoluble (P) fractions. Proteins in both fractions were resolved by SDS-PAGE, followed by Western blotting with anti-A3 (P4b/4b) and anti-A14 polyclonal antibodies and anti-HA MAb for G9. (C) Biotinylation of surface proteins. Purified virions prepared as in panel A were treated or mock treated with sulfo-NHS-SS-biotin. Excess biotin was quenched, and the virions were recovered by sedimentation, disrupted with SDS and DTT, and allowed to bind to a NeutrAvidin column, which was then washed. The unbound flowthrough (F) and the bound and eluted (E) materials were resolved by SDS-PAGE and analyzed by Western blotting with anti-HA MAb and anti-A4 and anti-A28 polyclonal antibodies.