Figure 2.
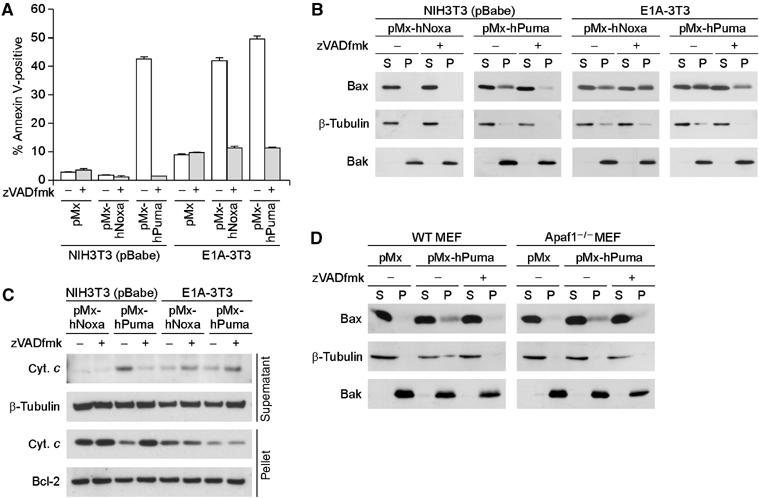
zVADfmk-sensitive and -insensitive pathways of MOMP induction. (A) Effect of zVADfmk on Puma- and Noxa-induced cell death in control NIH3T3 cells and E1A-3T3 cells. Under these experimental settings, zVADfmk treatment did not have clear cytotoxic effects. Values shown are means±s.d. from triplicate samples. (B, C) Inhibitory effect of zVADfmk on MOMP. The effects of zVADfmk treatment on membrane insertion of Bax (B) and cytochrome c release (C) were analyzed. In panel C, the amount of cytochrome c detected in the supernatant fractions of Noxa- or Puma- expressing E1A-3T3 cells was reproducibly increased by the zVADfmk treatment. Because the amount of cytochrome c detected in the pellet fraction decreased to a similar extent regardless of zVADfmk treatment, difference in the supernatant fraction is presumably due to the inhibitory effect of zVADfmk on the progression of apoptosis, that is, zVADfmk-treated cells do not readily lose their plasma membrane integrity and thereby they should retain released cytochrome c molecules in the cytosol for longer periods than untreated cells. (D) Puma-induced Bax membrane insertion in wild-type (WT) and Apaf1-deficient (Apaf1−/−) MEFs. WT and Apaf1−/− MEFs were prepared from the same litter. Notably, Puma-induced membrane insertion of Bax occurred in an Apaf-1-independent but caspase-dependent manner.
