Figure 1.
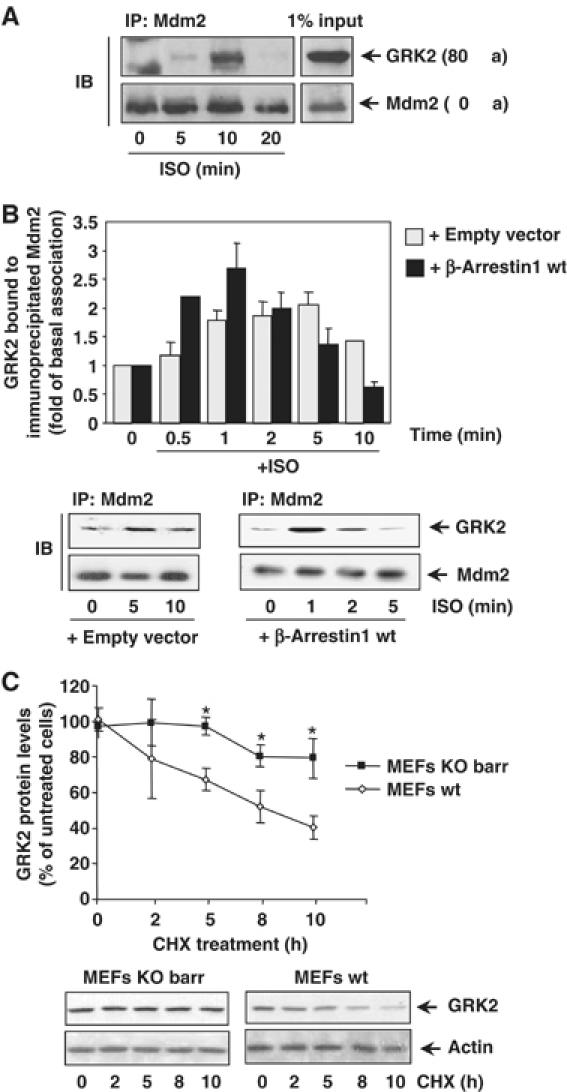
(A) Association of endogenous Mdm2 and GRK2. HeLa cells growing in 150 mm dishes were serum-starved for 2 h and incubated with an inverse β-agonist (betaxolol, 10 μM) for 15 min to lessen basal Mdm2/GRK2 association before challenging with 10 μM isoproterenol for the indicated times. Cells were lysed in buffer A as detailed in Materials and methods and lysates subjected to immunoprecipitation with anti-Mdm2 (SMP14) antibody. Immunoprecipitates were resolved by SDS–PAGE and GRK2 associated to the Mdm2 immunocomplex was detected by Western blot analysis with a specific antibody. 1% of the total lysate used for immunoprecipitation was loaded as input signal. (B) The Mdm2/GRK2 association is enhanced by β2AR stimulation and β-arrestin expression. HEK-293 cells were transiently transfected with plasmids encoding GRK2, β2AR, Mdm2 and β-arrestin1 when indicated. Cells were challenged with 10 μM isoproterenol for different times and GRK2/Mdm2 co-immunoprecipitation assessed as detailed in Materials and methods. GRK2 presence in the non-stimulated Mdm2 immunocomplex was taken as the control condition. Data were normalized by total Mdm2 and depicted as mean±s.e.m. of four independent experiments. A representative gel is shown in the bottom panel. (C) Normal decay of GRK2 protein is impaired upon of β-arrestin1 and β-arrestin2 knockdown. GRK2 protein levels were examined by immunoblot analysis in both wild-type and β-arrestin1/2-deficient MEFs upon treatment with cycloheximide (CHX) for the indicated times. The amount of GRK2 at 0 h was defined as 100%, and data normalized by actin protein levels. In both panels, data are the mean±s.e.m. of four independent experiments (*P<0.05). Representative gels are shown.
